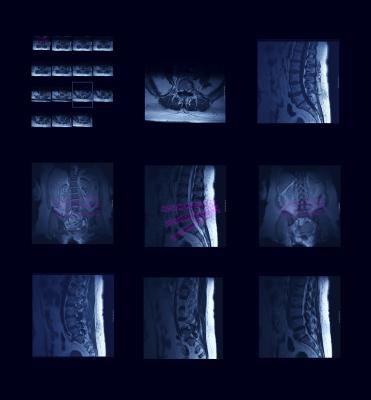
May 23, 2017 — Asterias Biotherapeutics Inc. recently announced new positive serial magnetic resonance imaging (MRI) data from its ongoing AST-OPC1 SCiStar Phase 1/2a clinical trial in patients with severe spinal cord injury.
Study data indicated that:
- For the five AIS-A patients in the SCiStar study treated with 10 million AST-OPC1 cells (Cohort 2) who also received a serial MRI scan at six months of follow-up, the serial MRI scans indicated no sign of lesion cavities in any patient. These fluid-filled cavities typically form by about three months following severe spinal cord injury and prevent significant recovery of motor and sensory function;
- For the three patients in Cohort 2 that have also completed 12 months of follow-up, serial MRI scans at 12 months continued to indicate no signs of lesion cavities; and
- All three AIS-A patients who received a low dose of 2 million cells (Cohort 1) in the SCiStar study also showed no sign of lesion cavities in any patient through 1 year of follow-up. All three patients are continuing long-term follow-up and will receive additional MRI scans annually.
The MRI results are supportive of the extensive pre-clinical data on AST-OPC1 showing that the cells durably engraft and help prevent cavitation at the injury site. Cavitation is a destructive process that occurs within the spinal cord following spinal cord injuries, and typically results in permanent loss of motor and sensory function. Additionally, a patient with cavitation can develop a condition known as syringomyelia, which results in additional neurological and functional damage to the patient.
Under the study protocol, patients are monitored by MRI scans at regular intervals over 12 months in order to assess status of the injection site and surrounding tissues.
For more information: www.asteriasbiotherapeutics.com


 February 13, 2026
February 13, 2026 









