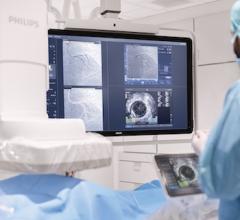
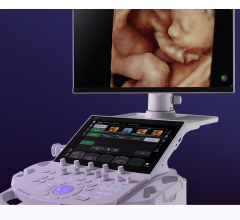
March 20, 2017 — Cardiologists can now access the advanced ultrasound imaging technology needed for fast and confident diagnoses with Toshiba Medical’s Aplio i900. The newly U.S. Food and Drug Administration (FDA)-cleared system is the latest addition to the premium Aplio i-series ultrasound platform with excellent imaging clarity and definition.
The Aplio i900 brings ultra-premium cardiac imaging to an everyday clinical setting, offering healthcare facilities a more cost-effective, less invasive and safer solution than other radiation-emitting modalities. The system offers innovative features that allow clinicians to quickly and easily assess myocardial function, or to quantify complex valvular lesions with greater depth and detail.
Ideally suited for contrast, fetal, pediatric, stress echo and transesophageal echocardiography imaging, according to Toshiba, the Aplio i900 delivers extreme processing power that helps healthcare providers see more in their ultrasound exams due to thinner beam slices and more return information in each image. A new beam-forming technology, iBeam, optimizes efficiency of the beam, increasing penetration, spatial resolution and contrast resolution. Boosting productivity during exams, the Aplio i900 provides intuitive ergonomics with iSense and touch control screens, and real-time quick scan makes automatic image adjustments without pushing a button.
Toshiba Medical showcased the Aplio i900 at this year’s American College of Cardiology (ACC) 2017 annual meeting in Washington, D.C., March 17–19, 2017.
For more information: www.medical.toshiba.com


 February 05, 2026
February 05, 2026