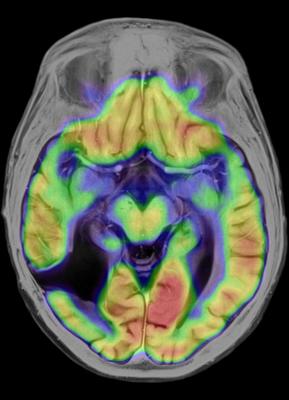
June 12, 2015 - Neuroinflammation caused by a reactive immune system could be tripping off the neurodegeneration seen in certain dementias, multiple sclerosis and other deadly diseases of the nervous system. A novel molecular imaging technique could be the key to understanding how best to treat these and other devastating diseases, according to a recent study presented at the 2015 Annual Meeting of the Society of Nuclear Medicine and Molecular Imaging (SNMMI).
At the heart of this maladaptive immune response are microglia, immune cells in the central nervous system that can be activated to trigger neuroinflammation. For this study, researchers used positron emission tomography (PET) to measure activation of microglia by employing a molecule from E. coli bacteria called lipopolysaccharide (LPS), or endotoxin. LPS stimulates the immune system and is accompanied by a radiotracer called carbon-11 PBR28 (C-11 PBR28). This form of molecular imaging allows the minimally invasive visualization of neuroinflammation. C-11 PBR28, is injected and binds to translocator proteins expressed on activated microglia. A PET scanner can then detect the radioactive particles emitted from inside the brain, representing areas of increased microglial activation before and after immune stimulation with LPS.
"The imaging technique could shed light on the immune dysfunction that underpins a broad range of neuroinflammatory diseases, such as Alzheimer's disease, depression, post-traumatic stress disorder and addiction," said Christine Sandiego, Ph.D., lead author of the study and a researcher from the department of psychiatry at the Yale School of Medicine in New Haven, Connecticut. "This is the first human study that accurately measures this immune response in the brain. The discoveries made with this technique could contribute to promising new drug treatments."
The PET radiotracer C-11 PBR28 was administered to eight healthy men around the age of 25, give or take six years, followed by two separate PET scans on the same day for each subject before and after injection with LPS. Adverse symptoms were self-reported and blood samples were taken to assess levels of peripheral inflammation. Results of the study showed that administering LPS led to a substantial spike in the systemic inflammatory response and levels of reported sickness, and activated microglia in the central nervous system.
With further research, eventual drug therapies could potentially cut the activation of neurodegenerative microglia and encourage neuroprotective processes in the brain.
For more information: www.snmmi.org


 February 13, 2026
February 13, 2026 









