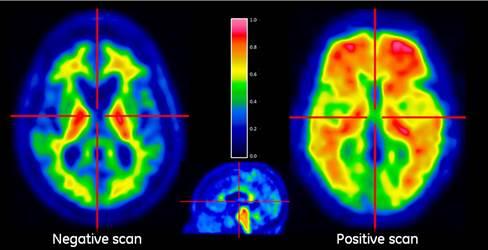
February 19, 2015 — One of the hottest topics in current Alzheimer’s research is brain scans that visualize tau pathology in living people. With the advent of positron emission tomography (PET) tracers that label neurofibrillary tangles, researchers are now able to "see" and quantify both hallmark pathologies defined by Alois Alzheimer in 1906: amyloid plaques and tau tangles. Tau tracers dominated the agenda when 340 researchers convened in Miami Beach, Florida, recently to debate the current edge in human amyloid imaging.
Previously, researchers could only detect plaques and tangles by examining brain tissue postmortem. PET also enables researchers to track pathology as people age. PET of amyloid plaques has been around for a decade, but tau PET is new.
Data is pouring in as academic and commercial labs test the current front-runner, T807/AV1451. The tracer is being validated in postmortem tissue and, as advertised, proves to bind phosphorylated tau aggregated into paired helical filaments. Researchers were excited to see the tracer bind in brain regions where neuronal metabolism crashes, and that this appears to match up with the symptoms that characterize both Alzheimer’s in its various forms and the frontotemporal dementias.
More work is needed to sort out the tracer’s technical features, but early indications of how A? and tau amyloids deposit as people age are widely considered to be highly promising. The field is getting crowded: New tau tracers debuted at the conference, and more are expected to come out soon.
For more information: www.alzforum.org


 February 13, 2026
February 13, 2026 









