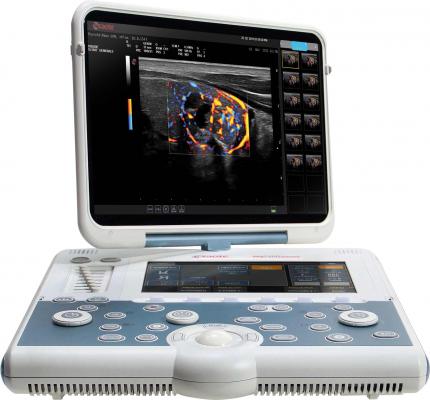
October 3, 2014 — Esaote North America announced that its new MyLab Gamma ultrasound system has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) and is now available for sale in the United States.
MyLab Gamma is a laptop-sized ultrasound system with lightweight portability and feature-rich applications that facilitate its use in a wide variety of clinical environments, from shared service to dedicated clinics and point-of-care.
“Today’s practices are under increasing pressure to deliver quality healthcare at an affordable cost,” said Larry Dentice, president and general manager of Esaote North America. “The portability and affordability of MyLab Gamma helps providers deliver excellent care wherever and whenever it’s needed."
It incorporates advanced clinical technologies to support comprehensive cardiac and vascular exams, including TEE (transesophogeal echo), strain, stress echo and other quantitative studies. With 3-D/4-D capability and a wide range of available probes, the system is also well suited to women’s health, general imaging and non-traditional point-of-care applications.
Ergonomically designed to be easy to use and reduce sonographer stress, MyLab Gamma can operate on battery power with fast boot times and rapid resume modes so it’s easy to transport and ready to use within seconds. The two onboard probe connectors can be expanded to four, with the optional cart, giving MyLab Gamma users access to a wide range of clinical utility and flexibility.
For more information: www.esaoteusa.com


 February 05, 2026
February 05, 2026 









