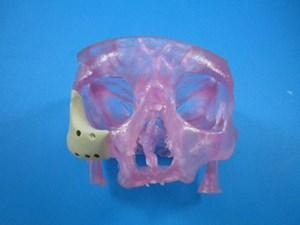
August 20, 2014 — Additive manufacturing (3-D printing) company Oxford Performance Materials (OPM) announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its 3-D printed OsteoFab patient-specific facial device.
OPM's facial device is the first and only FDA-cleared 3-D printed polymeric implant for facial indications, and follows FDA clearance of the first and only 3-D printed polymeric implant, OPM's OsteoFab patient-specific cranial device in February 2013.
"There has been a substantial unmet need in personalized medicine for truly individualized yet economical solutions for facial reconstruction, and the FDA's clearance of OPM's latest orthopedic implant marks a new era in the standard of care for facial reconstruction," said Scott DeFelice, CEO and chairman of Oxford Performance Materials. "Until now, a technology did not exist that could treat the highly complex anatomy of these demanding cases. With the clearance of our 3-D printed facial device, we now have the ability to treat these extremely complex cases in a highly effective and economical way, printing patient-specific maxillofacial implants from individualized MRI [magnetic resonance imaging] or CT [computed tomography] digital image files from the surgeon. This is a classic example of a paradigm shift in which technology advances to meet both the patient's needs and the cost realities of the overall healthcare system."
The facial device will be 3-D printed by OPM Biomedical, an OEM of medical devices utilizing the company's OsteoFab process, which combines laser sintering additive manufacturing technology and OPM's proprietary OXPEKK powder formulation to print orthopedic and neurological implants. These implants are biocompatible, mechanically similar to bone, radiolucent and support bone attachment (i.e., osteoconductive).
OPM technology is also designed to reduce the overall "cost of ownership" to the customer by decreasing operating room time, hospital length of stay and procedure complications. In addition, OsteoFab customers do not pay a premium for the individualized 3-D printed implant.
"An exciting aspect of our technology is that additional complexity does not increase manufacturing cost, and having both cranial and facial devices cleared now enables us to answer ever more complex cases where upper facial structures can be incorporated with cranial implants as a single device," added Severine Zygmont, president of OPM Biomedical. "As a result, additive manufacturing has the potential to not only improve patient outcomes, but fundamentally improve the economics of orthopedics on a global scale — for developed and developing countries. These are disruptive changes that will allow the industry to provide the finest levels of healthcare to more people at a lower cost."
Biomet Inc., a distributor of advanced technologies for the treatment of arthritis, joint and spine related injuries and facial reconstruction, will be the exclusive global distributor of OPM's facial device. Biomet is also the exclusive global distributor of OPM's cranial device.
For more information: www.oxfordpm.com


 February 06, 2026
February 06, 2026 









