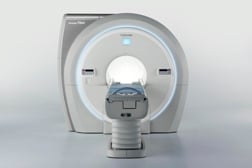
The new Vantage Titan 1.5T magnetic resonance (MR) series, pending 510(k) clearance, is a scalable solution with a full upgrade path from the 8-channel to the 16- or 32-channel system. Features include a 71 cm bore, Pianissimo noise reduction technology, advanced noncontrast imaging, integrated coils with Atlas Speeder technology and an intuitive M-Power user interface.
For advanced cardiac imaging, the line is upgradeable to a 16- or 32-channel system with high slew rate gradients, which enable higher spatial and temporal resolution.
Toshiba America Medical Systems Inc. showed the system at AHRA 2012.
For more information: www.medical.toshiba.com


 February 16, 2026
February 16, 2026 









