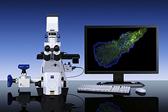
Carl Zeiss MicroImaging Inc., a leading provider of microscopy solutions for a variety of research, clinical and industrial applications, introduces its Laser TIRF 3 microscope system, designed to enable visualization of near-cell membrane dynamic processes while maintaining optimum specimen incubation conditions.
The Laser TIRF 3 also allows observation of single molecule dynamic processes in cell-free systems and, in combination with other techniques such as atomic force microscopy (AFM), the new microscope provides a complete solution for users in the life sciences, biochemistry, molecular biology and biophysics arenas.
The Laser TIRF 3 maintains Carl Zeiss' long-standing commitment to system flexibility. A range of incubation options maintain viable conditions for live cell experiments. Together with the 'definite focus' module, users can be assured of accurate quantitative data over long time periods. The new laser module may be equipped with up to four solid-state lasers, is AOTF-controlled (acousto-optical tuneable filter) and may be operated entirely from the AxioVision software interface.
The TIRF slider is available in two versions; either manual or fully-motorized and software controllable. The motorized version permits a given illumination angle to be set with significantly greater accuracy and speed than other current systems and the reproducible angle setting results in reproducible penetration depths for the light beam. Together with the corrected beam-path and special filter sets, the apochromatically-corrected optics of the TIRF slider guarantee maximum image quality.
AOTF control and angle setting are integrated into the 'fast image acquisition' module of the AxioVision software, enabling significantly more high resolution images to be acquired within any given timeframe.
The new Laser TIRF 3 builds on the attributes of the Laser TIRF, the first microscope to offer the combination of TIRF and transmitted-light contrasting techniques, such as DIC and brightfield, and enable the sequential recording of image pairs. By selectively exciting cellular fluorophores adsorbed, adhered, or bound to the surface and combining it with conventional epi-fluorescence, researchers can relate surface effects to internal cellular structures.
For more information: www.zeiss.com/micro


 February 20, 2026
February 20, 2026 









