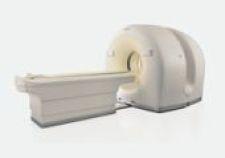
The Philips BrightView XCT integrates Philips BrightView SPECT in a co-planar design with advanced flat-detector X-ray CT technology to acquire low-dose, high-resolution CT images and to improve registration confidence. The system represents the first time a flat panel X-ray detector will be used for CT imaging in nuclear medicine.
The BrightView XCT features technological advances that can enable low patient dose levels, high-resolution localization and high-quality attenuation correction with the potential for fewer artifacts and shorter exam times. This offers clinical advantages particularly in cardiology studies, the top procedure in nuclear medicine. In addition, the co-planar SPECT and CT capabilities limit, and in some cases eliminate, the need to move the table between scans. Reduced movement can help improve patient comfort and allow for more confidence in image registration, the process of comparing, matching and superimposing the SPECT and CT images on one another for analysis. The BrightView XCT is also the only scalable SPECT and SPECT/CT system that fits into a 12-inch by 15.5-inch room and does not require special certification for nuclear medicine technicians.
Additionally, Philips will showcase the new GEMINI TF PET/CT and GEMINI TF PET/CT Big Bore, which features 4D time-of-flight technology, a toolkit to better manage respiratory motion by offering comprehensive tools for combined CT, PET and PET/CT correlated imaging.
The new Philips GEMINI TF Big Bore is the first commercial Big Bore PET/CT, with a full 85 cm bore diameter for both PET and CT scans. The system combines Philips time-of-flight PET imaging technologies with its Brilliance CT Big Bore simulation to optimize oncology workflow, accuracy and patient experience. The GEMINI TF PET/CT Big Bore system is designed to meet the requirements of radiation oncology applications, providing improved planning in cases that call for patients to be in extended positions, such as with treatments of breast and colorectal cancers. The system allows patients to be positioned for simulation in the same manner as they would to receive therapy.


 October 30, 2025
October 30, 2025 









