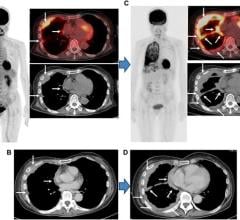
February 7, 2007 - Siemens Medical Solutions today opened the doors to a new molecular imaging biomarker research site, Siemens Medical Solutions Molecular Imaging (MI) Biomarker Research facility, as Siemens strives to become the world's first full-service diagnostics company, integrating in vivo and in vitro imaging diagnostics capabilities.
At the new facility, researchers will be dedicated exclusively to the development of molecular imaging biomarkers. The goal of the research is to spur the growth of in vivo molecular diagnostics, in which in vivo diagnostic tools are designed to identify debilitating diseases such as cancer and neurological diseases at their earliest stages. The biomarkers bind to the diseased cells or tissues and cause them to "light up" when scanned using PET-CT (Positron Emission Tomography-Computed Tomography) or SPECT-CT (Single-Photon Emission Computed Tomography).
Siemens' goal is to bring several new agents to the market over the next 5 - 10 years. Research and development efforts conducted at the facility will focus
largely on oncology and neurology, and also include other areas such
as inflammation and microfluidics/nanotechnology research.
"Molecular medicine is heralding a new era in diagnostic capabilities that could change the lives of millions of Americans, and Siemens is helping lead the field out of the research lab and into practical use," said Michael Reitermann, president, Molecular Imaging division, Siemens Medical Solutions. "Advancing this field brings with it the promise of personalized therapeutics, which would not only improve the efficiency of health care, but most importantly, would also improve the quality of health care for patients."
The opening of the site is the latest step for the company in becoming Siemens recently launched Siemens Medical Solutions Diagnostics as the in vitro complement to the portfolio on the heels of acquisitions of Bayer Diagnostics and Los Angeles-based Diagnostic Products Corporation.


 January 27, 2026
January 27, 2026