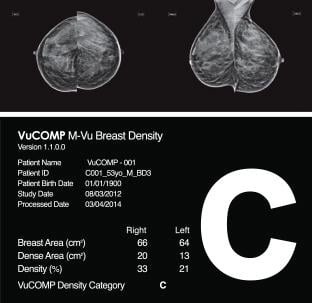
July 31, 2014 — VuCOMP announced it has amended its Health Canada medical device license to include M-Vu Breast Density. The amendment provides clearance for VuCOMP to sell and market its M-Vu Breast Density product throughout Canada.
The clearance is timely as bill C-314, an act respecting the awareness of screening among women with dense breast tissue, is introduced in Canada. The act requires the Canadian government to encourage the use of existing initiatives in order to increase awareness among women about the implications of heterogeneous or dense breast tissue for breast cancer screening, and to assist women and healthcare providers in making well-informed decisions regarding screening.
Jim Pike, president and CTO of VuCOMP, said, “We are pleased to now offer M-Vu Breast Density to the breast radiology community in Canada. Breast cancer is the second leading cause of death from cancer in Canadian women, with over 24,000 women diagnosed each year. We hope our M-Vu Breast Density tool will aid radiologists as they fight to lower this statistic.”
Clinical studies have shown that dense breasts can hide a cancerous lesion by reducing the ability to visualize fine structures and details that could indicate a malignant abnormality, making breast cancer detection in a mammogram more difficult. M-Vu Breast Density is designed to advance the science of breast density measurement by identifying dense tissue in the breast that could be masking cancer.
For more information: www.vucomp.com


 February 18, 2026
February 18, 2026 









