Photo courtesy of Volpara Solutions
Volpara Solutions will exhibit its complete suite of quantitative breast imaging tools built on the Volpara Solutions software engine that allows for personalized measurements of volumetric breast density, patient-specific dose, breast compression and other factors designed to maintain accuracy and consistent quality in breast screening.
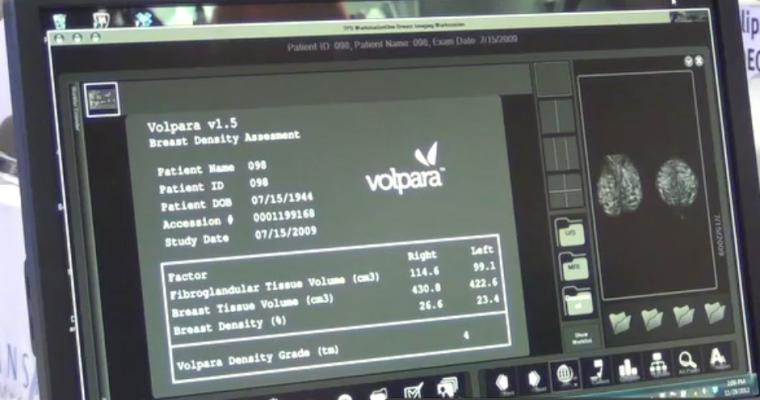
Cleared by the FDA, HealthCanada, the TGA and CE-marked, VolparaDensity is used by radiologists to objectively assess density from both digital mammography and tomosynthesis images to help doctors evaluate which women would benefit from additional screening. Highly correlated to 3-D breast MR assessments, VolparaDensity is a reliable tool that automatically generates an objective measurement of volumetric breast density correlated to the ACR (American College of Radiology) breast density categories. To date, nearly 4-million women have had their breast density analyzed using VolparaDensity.
VolparaAnalytics assists in quality assurance by monitoring critical elements of the breast imaging process and generating key metrics to help breast imaging centers monitor the performance of technologists, readers, and mammography and tomosynthesis machines. VolparaAnalytics provides unique information such as average pressure applied by each technologist, and breast density profiles by mammography system, helping address potential issues early.
VolparaDose calculates mammography dose by using a standard mean glandular dose (MGD) algorithm along with patient-specific volumetric breast density to give a better estimate of the dose absorbed by the specific breast. The VolparaDose data can be inserted directly into patient letters on compatible mammography reporting systems, and sent to enterprise wide dose tracking systems.
For information: visit www.volparasolutions.com


 February 18, 2026
February 18, 2026 









