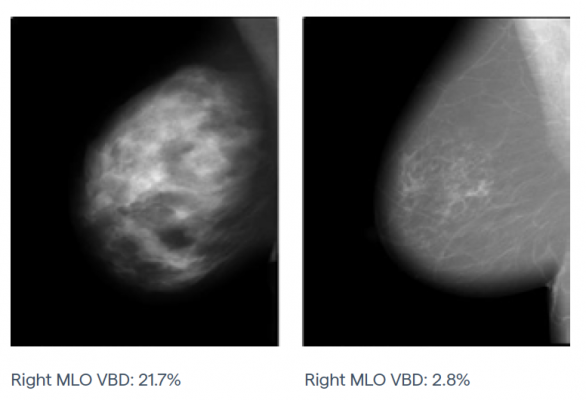
Right mediolateral oblique (MLO) mammograms for different women with the same breast thickness but varying breast density.
July 30, 2021 — Volpara Health, a global health technology software leader providing an integrated breast care platform for the delivery of personalized breast care, has received FDA clearance for the latest version of its key science algorithm, Volpara Imaging Software (VIS 3.2).
VIS 3.2 improves the overall robustness of Volpara's industry-leading breast density assessment algorithm, incorporating learnings from artificial intelligence (AI). The clearance also expands the use of VIS 3.2 to additional mammography machines, increasing support for various Giotto and Siemens units, thus extending Volpara's reach and multi-vendor capabilities.
Since receiving initial FDA clearance for Volpara Imaging Software in 2010, Volpara has now received four additional clearances for the key science algorithm behind its integrated Volpara Breast Health Platform—a product suite designed to enable earlier detection of breast cancers through improved mammography quality and workflow, volumetric assessment of breast density, and personalized breast care. Volpara's technology is protected by 98 patents worldwide and is validated by more than 375 peer-reviewed publications and research abstracts.
The latest clearance includes Volpara's Open Virtual Appliance (OVA) architecture, which increases image processing security and makes it easier for Volpara to monitor, service, and update software services—a key part of the company's strategic move to achieve greater scalability and lay the foundations for future new products.
"These new innovations improve the overall security, scalability, robustness, and breadth of our breast health offering. Our objective with each enhancement is the pursuit of our mission—to eliminate advanced-stage breast cancer and save more families from cancer," said Ralph Highnam, Ph.D., Group CEO of Volpara.
For more information: www.volparahealth.com


 March 09, 2026
March 09, 2026 









