September 24, 2013 — Cancer patients receiving radiotherapy (RT) who are potentially suffering from depression can be effectively identified by a two-item questionnaire, according to research presented at the American Society for Radiation Oncology’s (ASTRO’s) 55th Annual Meeting.
The Radiation Oncology Therapy Group (RTOG) Community Clinical Oncology Program (CCOP)-supported multi-institutional clinical study screened 455 patients receiving radiation treatment at 37 centers around the United States. Participants in the study were seeking treatment for breast cancer (45 percent); GI cancer (11 percent); lung cancer (10 percent); gynecologic cancer (6 percent); or other cancers (27 percent). Sixty-six percent of the patients in the trial (298) were women.
Depression screenings were performed before or within two weeks of treatment of the initial cancer diagnosis. The screening forms included the single-item National Comprehensive Cancer Network-Distress Thermometer (NCCN-DT); the Hopkins Symptom Checklist (HSCL-25); and the nine-item Patient Health Questionnaire, (PHQ-9), which includes Patient Health Questionnaire-2 (PHQ-2) as its first two questions. All of the study participants answered the screening questionnaires with 100 percent completion.
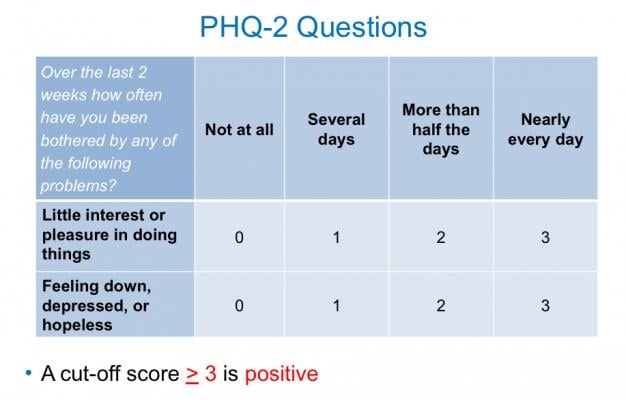
Patients received the PHQ-9 and were asked if, within the past two weeks, they had “little interest or pleasure in doing things,” or “if they were feeling down, depressed or hopeless.” It was discovered that patients’ responses to these two questions (the PHQ-2) were as useful in identifying depression as results from the entire PHQ-9, and were more indicative than results from the NCCN-DT.
Within the study, a total of 75 patients (16 percent) screened positively for depressive symptoms. PHQ-9 and PHQ-2 had similar accuracy in detecting depression with an area under the curve (AUC) of approximately 0.83 for each and was superior to the HLSC-25 (.79) and the NCCN-DT (.60).
Of the facilities included in the study, 68 percent offer mental health services. Patients who screened positive for depression symptoms, along with a systematic sample of patients who screened negative, were administered the Structured Clinical Interview for DSM-IV (SCID) Mood Disorder modules by telephone. The study determined that screening in an RT setting was well received by patients and feasible.
“Detection of depression in cancer patients is an important public health priority, and the ability to screen and treat cancer patients for depression can have a major impact on a patient’s quality of life,” said William Small Jr., M.D., FASTRO, presenting author of the study and chairman of the Department of Radiation Oncology at Loyola University Chicago. “This study was designed to test the feasibility of screening for major depression in cancer patients receiving radiation therapy.
The ability of a two-question survey to effectively screen for depression will hopefully prompt more centers to screen and to refer patients in need of mental health services,” said Lynn I. Wagner, Ph.D., principal investigator of the study and an associate professor in the Department of Psychiatry and Behavioral Sciences at the Northwestern University Feinberg School of Medicine and the Robert H. Lurie Comprehensive Cancer Center at Northwestern University in Chicago.
The abstract, “RTOG 0841: Two Item Questionnaire Effectively Screens for Depression in Cancer Patients Receiving Radiotherapy,” was presented at ASTRO’s Annual Meeting in the Plenary session Sept. 23.
For more information: www.astro.org


 January 30, 2026
January 30, 2026 









