August 8, 2008 - TomoTherapy Inc. will introduce the TomoDirect discrete-angle delivery mode for the TomoTherapy Hi-Art treatment system at European Society for Therapeutic radiology and Oncology (ESTRO) in Gothenburg, Sweden, Sept. 14-18, 2008, and the American Society for Therapeutic radiology and Oncology (ASTRO) in Boston, MA, Sept. 21-25, 2008.
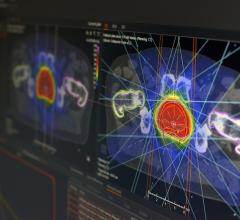
This new technology allows Hi-Art system users to quickly plan and deliver advanced TomoTherapySM radiation treatments with a series of linear beam paths, rather than the existing helical path, said the manufacturer. With TomoDirect, clinicians can choose several discrete angles and the optimal modulation level required for delivery, based on specific patient therapy goals.
TomoDirect was developed as a complement to helical TomoTherapy, with both utilizing the same binary multi-leaf collimator and CT-style gantry technology. The choice of which modality to use for a given case will depend on the nature of the tumor volume and surrounding organs at risk. TomoDirect is expected to provide time savings in both the planning and delivery phases for several clinical scenarios, including whole breast irradiation and palliative treatments.
TomoDirect research partner Paul Read, M.D., Ph.D., assistant professor of Radiation Oncology, University of Virginia, said, "TomoDirect will expand the spectrum of external beam radiation therapy patients who can be optimally treated with TomoTherapy's unique image-guided, intensity-modulated radiation therapy (IG-IMRT). Now, we will have an option to deliver dose via two unique and complementary delivery modes, choosing whichever provides the optimal dose distribution. Clearly, breast cancer patients will benefit from TomoDirect, and other disease sites will likely be developed for which there is clinical benefit."
Added research partner Prof. Guy Storme, M.D., Ph.D., director of the Oncologic Center at UZ Brussel, Brussels, Belgium: "Certainly, with the combination of helical and direct deliveries, TomoTherapy should offer significant benefit for a majority of breast cancer treatments. It will provide the ability to choose and apply the right modality for the best treatment. Our research is focused on providing comprehensive usage guidelines as we study and validate this new mode combination."
In addition to the added capabilities offered by TomoDirect, the Hi-Art system's treatment modes are being expanded to include a 3D conformal option, thereby meeting the full range of options needed for all clinical cases.
TomoTherapy is planning to make TomoDirect available to radiation clinics in summer 2009. As a product pending FDA 510(k) clearance, TomoDirect is not yet available for sale in the U.S.
For more information: www.tomotherapy.com


 June 19, 2024
June 19, 2024