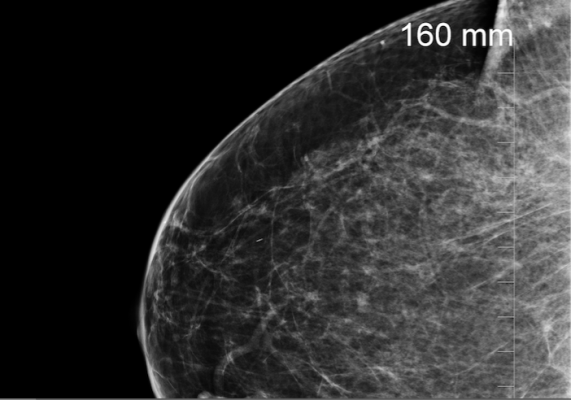
June 23, 2017 — French radiology technology company Therapixel was recently named the winner of the Digital Mammography Challenge for their artificial intelligence algorithm designed to enhance mammography reading.
Breast cancer affects one in eight women during their lifetime. Early detection is effective in controlling the disease, with a five-year survival rate greater than 99 percent. However, systematic screening leads to the recall of 10 percent of patients for a complementary biopsy examination. Only 5 percent of the recalled patients are actually affected by the disease.
The Digital Mammography Challenge aims, in the form of a global competition, to improve the performance of screening by exploiting artificial intelligence algorithms. The 2017 Challenge featured more than 1,200 participants and 640,000 anonymous exams. Entries were judged across four rounds over nine months.
Therapixel’s winning algorithm improves the rate of false positives by 5 percent compared to the state of the art, according to the company.
For more information: www.therapixel.com


 February 13, 2026
February 13, 2026 









