RS80A ultrasound image courtesy of Samsung
January 6, 2016 — Samsung Electronics debuted the newest additions to its medical imaging portfolio at the 2015 Radiological Society of North America (RSNA) annual meeting, Nov. 29-Dec. 3 in Chicago. The range of solutions included computed tomography (CT), ultrasound and digital radiography (DR) systems.
Samsung’s CT imaging solutions debuting at RSNA included:
CT
NExCT 7 – The first premium CT scanner from Samsung features high resolution designed to improve diagnostic confidence in advanced and routine exams reducing the radiation dose as low as reasonably achievable and ensuring a fast and efficient workflow. Some features of the NExCT7 are U.S. Food and Drug Administration (FDA) 510(k) pending.
Portable CT – Samsung showcased the latest in software and hardware enhancements across its portable CT product line. These upgrades improve user satisfaction and streamline workflow, and both systems are now compliant with the Smart Dose (XR-29) radiation dose reporting standard.
The BodyTom and CereTom portable systems continue to prove their versatility as they are increasingly adopted and utilized in a wide range of market applications such as mobile stroke units, brachytherapy suites, proton therapy centers, deep brain stimulation and even veterinarian hospitals.
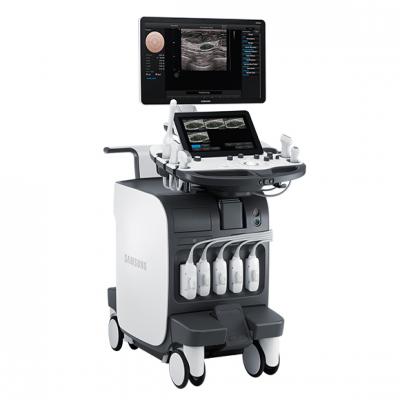
Ultrasound
RS80A with Prestige – Now available in the U.S. market, this system features advanced technical capabilities built on Samsung technologies. Product features include:
- S-Fusion to enable simultaneous localization of lesions with a real-time ultrasound image supported by other modalities' 3-D data sets. Fusion speed and accuracy are the strengths of S-Fusion.
- S-Shearwave to detect the velocity of the shearwave transmitted through the targeted lesion and display the numerical measurement of stiffness.
- E-Breast*: This is the function for breast lesion exam, which calculates the strain between the area of the suspected lesion (ROI) and normal breast fat and displays the results.
- E-Thyroid*: This function assists with the diagnosis of thyroid lesions. The ROI is used to show the contrast of an elastogram.
*The strain ratio value is a relative value regarding quantification, not an absolute value.
HS70A – Designed for hospital and private care. This system has a flexible architecture to meet the clinical needs of varied users. The HS70A offers a complete radiology and cardiovascular solution, including technologies migrated from the premium radiology RS80A platform. Features include S-Detect, which uses Breast Imaging-Reporting, and Data System (BI-RADS) scores for analysis and classification of suspicious lesions.
DR
GC85A – A DR system offering streamlined workflow, diagnostic confidence and improved total cost of ownership. The GC85A features imaging engine S-Vue, ensuring consistency with high quality, convenient setting of contrast and improved sharpness and clarity, S-Share to support unique clinical needs and continuous care with increased connectivity, and Smart Control for one-touch precise positioning.
GM60A – A mobile DR system that delivers high-quality imaging directly to the patient’s bedside. Advanced mobility allows use in the patient room, emergency room, operating room and the intensive care unit, and has advanced features, including S-Detector and S-Vue imaging technologies to deliver accurate diagnoses while ensuring reliable operation with enhanced usability. Additionally, the telescopic column ensures fast navigation by securing a clear view for users.
For more information: www.samsung.com


 March 06, 2026
March 06, 2026 









