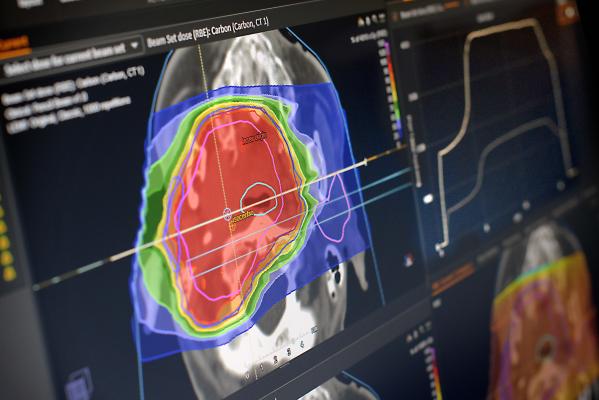
November 20, 2017 — Provision Healthcare has selected RayStation for external beam planning at the new Provision Cares proton therapy center in Franklin, Tenn., which is currently under construction. The center forms part of the Provision Cares Cancer Network.
The center, which is scheduled to open in 2018, will be the first clinical ProNova proton system in the world, featuring superconducting magnets and advanced imaging capabilities. The new ProNova SC360 machine will be fully supported by both RayStation and RayCare, RaySearch’s new oncology information system, which will be submitted for U.S. Food and Drug Administration (FDA) clearance at the end of 2017.
RayStation will be used for all external beam treatment planning at the center. The installation will feature a range of advanced functionalities, such as adaptive planning, deformable registration and fallback planning, which allows planning comparisons between modalities.
David Raubach, vice president of business development at Provision Healthcare, said: “The plan from the beginning was to make this one of the most advanced proton treatment centers in the world from a technology perspective. We are delighted that RaySearch has partnered with us to provide full support for the first and only 360-degree compact system. And we are confident that the combination of RayStation and RayCare with the new ProNova system will provide a unique platform that will set the new standard for effective and efficient treatment processes.”
For more information: www.raysearchlabs.com


 January 30, 2026
January 30, 2026 









