March 1, 2016 — Queen’s University Belfast, in partnership with the Belfast Trust, is leading the world’s first ever trial of a new combination of cancer therapies for patients with advanced prostate cancer, with the hope of prolonging their lives.
The ADRRAD trial, which recently started at the Northern Ireland Cancer Centre, is funded by and supported by Friends of the Cancer Centre and Bayer Pharmaceuticals.
In 2014 there were over 1,100 newly diagnosed cases of prostate cancer in Northern Ireland. Almost 8,500 men there are living with a diagnosis of prostate cancer, and just under 250 men each year die as a result of the illness.
Thirty patients will participate in the trial over the next 18 months. It is aimed at men with advanced prostate cancer, where the cancer has spread to the bones at the time of diagnosis. This accounts for around 10 percent of prostate cancer patients.
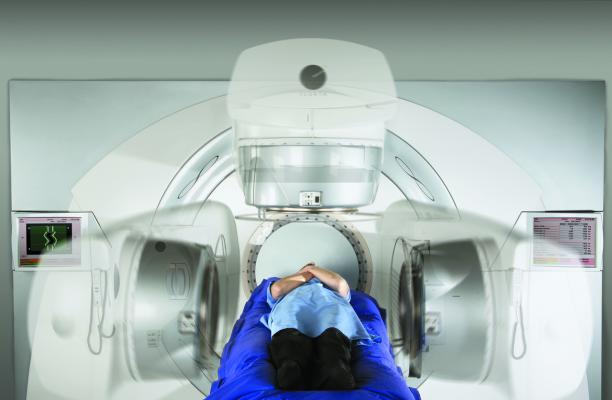
Men with advanced prostate cancer are normally treated with hormone therapy, which aims to shrink a tumor by limiting the amount of testosterone reaching the cancer cells. The new approach being trialed by Queen’s researchers is the first to combine two existing forms of radiotherapy — volumetric modulated arc therapy (VMAT) to target prostate cancer cells in the pelvis, along with radium 223 to target the disease in the bones. If successful, it has the potential to completely change the way in which the disease is treated and potentially extend the life expectancy of patients with the advanced stages of the disease.
VMAT is an advanced type of radiation therapy which manipulates radiation beams to conform to the shape of a tumor, delivering precise radiation doses to a tumor, while minimizing the dose to surrounding normal tissue. It is delivered externally, using a radiotherapy machine called a linear accelerator
Radium 223 is a relatively new ‘bone-seeker’ drug. It is a type of internal radiotherapy, which is given intravenously. Once it is in the bones, radium 223 releases radiation which travels a very short distance — between 2 and 10 cells deep, which is less than a millimeter. This means it delivers a high dose of radiation close to the tumor deposits in the bone, killing the cancer cells and minimizing damage to the healthy cells.
Prof. Joe O’Sullivan from Queen’s University’s Centre for Cancer Research and Cell Biology and clinical director of oncology in Belfast Trust is leading the trial at the Northern Ireland Cancer Centre at Belfast City Hospital. He said: “This is the first trial of its kind anywhere in the world. It is hoped that combining the two forms of radiotherapy will be more effective than existing hormone treatment in targeting prostate cancer cells at multiple sites and extend the life expectancy of men whose treatment options are otherwise limited. We expect results from the initial trial within two years, with the view to then embarking on a larger trial with a greater number of patients.
“This trial is a crucial development in the fight against prostate cancer, which is the most common type of cancer among men in Northern Ireland. Three men here are diagnosed with this disease every day. Thousands are living with the illness, which unfortunately claims one life every hour across the U.K. Queen’s, Belfast Trust and Northern Ireland are at the forefront of global efforts to develop more effective treatments for all types of cancer. The ADRRAD trial is an excellent example of the potentially life-changing and life-saving impact of this work.”
The ADRRAD trial is sponsored by Belfast Health and Social Care Trust. Scientific work which takes place alongside the trial is supported by Prostate Cancer UK and the Movember Foundation as part of the Belfast-Manchester Movember Centre of Excellence – a partnership between Queen’s and the University of Manchester.
Iain Frame, M.D., director of research at Prostate Cancer UK said: “This trial represents a really exciting shift in how we think about prostate cancer – away from aiming to prolong life for men with advanced prostate cancer, towards taking the first steps to stopping the disease in its tracks once and for all. The scale of what we can achieve when we work together as funders, clinicians, scientists and men must not be underestimated. We are on the brink of remarkable breakthroughs in prostate cancer research, and this trial could be one of them.
“That’s why we mustn’t falter now. If we continue investing in world class research like this, within ten years, the world of prostate cancer research and treatment will be a far more hopeful place for men with and at high risk of the disease.”
For more information: www.qub.ac.uk


 January 30, 2026
January 30, 2026 









