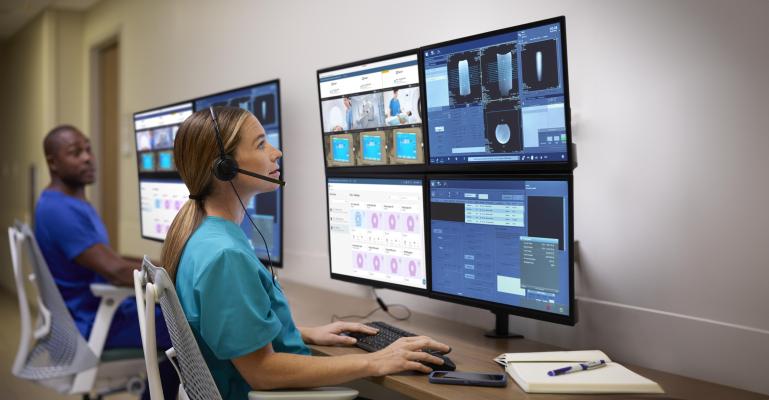
Nov. 22, 2024 — Royal Philips recently announced it had received U.S. Food and Drug Administration (FDA) 510(k) clearance of remote scanning and remote protocol adjustment features on Philips Radiology Operations Command Center (ROCC) [1]. This means radiologists can now assist technologists on-the-spot, by remotely controlling scans or adjusting protocols to acquire high quality images needed for improved diagnostic confidence and patient outcomes.
“With the ROCC, innovation takes the lead in revolutionizing our daily radiology operations, enhancing our imaging capabilities, and providing staffing flexibility, enabling us to ultimately serve more patients more efficiently, overall delivering better patient care,” said Sherri Lewman, senior vice president of Enterprise Imaging at Tampa General Hospital [2].
ROCC is a multi-vendor, multi-modality, safe and secure imaging solution widely used in North America and Europe. It can be used with any vendor MR or CT configuration and seamlessly connects imaging experts in a command center with technologists at scan locations across their organization. A pilot study at Imperial College Healthcare NHS Trust in London, UK resulted in a 9% increase in total scanning throughput as a result of reduced scan times and a zero exam recall rate over the study period [3].
The remote scanning capabilities of ROCC [1] allow expert users to edit MR and CT scanner consoles in real time, from any location and share their expertise via on-demand chat, voice and video collaboration, without compromising privacy, safety or security. ROCC enables remote collaboration between imaging experts and on-site technologists to help with precise, first-time right image acquisition, for more efficient and enhanced patient care.
“Healthcare providers are increasingly confronted with the challenge of not having enough skilled technologists to meet the demand for patient imaging exams, particularly for more complex exams such as cardiac MR,” said Shiv Gopalkrishnan, Business Leader Patient Care Informatics at Philips. “With the 510(k) clearance of ROCC’s remote scanning and remote protocol management capabilities [1] we are further empowering clinicians to deliver the timely diagnosis that patients deserve and helping to deliver better care for more patients.”
Philips will showcase ROCC at the Radiological Society of North America Annual Meeting, Dec. 1-4, 2024, in Chicago, USA.
For more information, visit Philips at RSNA 2024.
[1] Remote editing and protocol management are functions powered by the 510(k) cleared ROCC Console solution. Remote Image Acquisition is only to be used with a qualified user at the scanner.
[2] TGH Imaging Implements Philips Radiology Operations Command Center (ROCC) | Tampa General Hospital
[3] Based on results achieved in the ROCC Connectivity-Imperial NHS Trust Pilot Study. Increasing Imperial-NHS cardiac scanning capacity using ROCC for virtual training white paper. September 2024. Results are specific to the institution where they were obtained and may not reflect the results achievable at other institutions.


 February 27, 2026
February 27, 2026 









