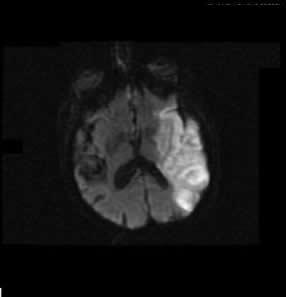
July 29, 2011 — Oxygen Biotherapeutics Inc. and privately held Aurum Biosciences Ltd. of Glasgow, Scotland, have signed a letter of intent (LOI) to conduct preclinical research for imaging and therapeutic intervention of acute ischemic stroke. Aurum, using Oxygen’s proprietary Oxycyte PFC (perfluorocarbon) emulsion in combination with Aurum’s proprietary Glasgow Oxygen Level Dependent (GOLD) magnetic resonance imaging (MRI) techniques, will conduct the research.
The intent is to better delineate which tissue has been damaged from a stroke, and to determine the effectiveness of the treatment being provided to the model. Per the LOI, Aurum will seek funding for this research.
An ischemic stroke occurs when a blood vessel that carries oxygen and nutrients to the brain is blocked by a clot. When that happens, part of the brain cannot get the blood and, therefore, the oxygen it needs, so it starts to die.
According to the American Stroke Association (ASA), approximately 795,000 Americans each year suffer a new or recurrent stroke. That means, on average, a stroke occurs every 40 seconds. Stroke kills more than 137,000 people a year in the United States alone and is the third leading cause of death behind diseases of the heart and cancer. On average, someone dies of stroke every four minutes. About 40 percent of stroke deaths occur in males, and 60 percent in females.
According to The Stroke Association, an estimated 150,000 people have a stroke in the United Kingdom each year, accounting for around 53,000 deaths annually. Stroke is the third most common cause of death in England and Wales, after heart disease and cancer. It accounts for 9 percent of all deaths in men and 13 percent of deaths in women in the United Kingdom.
For more information: www.oxybiomed.com


 February 13, 2026
February 13, 2026 









