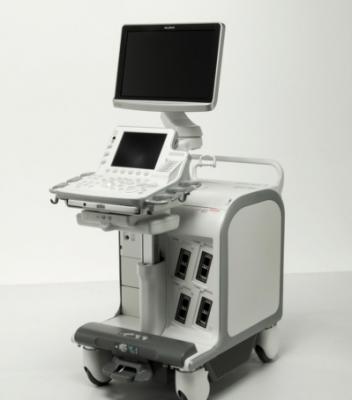
May 15, 2013 — Olympus announced the company's ultrasound endoscopes will now be powered by the ProSound F75 Ultrasound Processor.
The ProSound F75 Ultrasound Processor, developed in partnership by Hitachi Aloka Medical Ltd., provides physicians with the highest quality imaging that aids in more accurate diagnosis of diseases of the gastrointestinal (GI) tract and surrounding organs such as the pancreas, bile duct, liver, spleen and gallbladder as well as assessing a variety of cancers. The newly introduced platform improves workflow from pre-examination to after examination for better data management and provides improved visualization for more accurate blood flow information.
"The ProSound F75 delivers outstanding image quality that facilitates easy identification and more accurate staging of GI tumors in my practice," said Shyam Varadarajulu, M.D., medical director of the Center for Interventional Endoscopy (CIE) and professor of medicine at the University of Central Florida. "The images are crisp with excellent resolution and the technology is truly versatile in its functionality."
The ProSound F75 Ultrasound Processor offers an ergonomic capability with an adjustable height and depth of the keyboard and monitor that provides comfort to all endoscopists in a practice. The ProSound F75 has versatile capabilities for conducting quick tasks and examinations in every clinical setting. In addition, the device is forward and backward compatible, which allows it to work with most endoscopic ultrasound (EUS) and endobronchial ultrasound (EBUS) scopes.
EUS combines ultrasound technology with endoscopy to better visualize the tissues of the digestive tract and adjacent anatomical structures inside the human body. Conventional ultrasound is performed by placing a transducer against the skin to produce images of internal organs. With EUS, the transducer is endoscopically inserted into the body via the digestive tract, putting it closer to the area of interest to obtain higher resolution images.
This technology addresses the key requirements of Healthcare Reform:
- Increased Quality of Care – EUS drives optimal patient outcomes through easy identification and more accurate staging of diseases and cancers in the GI tract.
- Decreased Costs – The forward and backward compatibility provides significant cost savings through lower cost of ownership and more efficient use of assets.
- Enhanced Patient Satisfaction – EUS provides a less invasive approach than conventional surgical diagnosis allowing physicians to view internal anatomy without making an incision.
For more information: www.olympusamerica.com, www.hitachi-aloka.com


 February 05, 2026
February 05, 2026 









