May 13, 2024 — Avenda Health, an AI healthcare company creating the future of personalized prostate cancer care, unveils the results of a new UCLA study examining the role of AI in identifying tumor margins and predicting the efficacy of focal therapy treatments. The study titled “Software to Determine Extent of Tumor Margins in Focal Therapy,” was presented at the 2024 American Urological Association’s (AUA) annual meeting by Wayne Brisbane, MD, assistant professor at UCLA Health. It demonstrated that Unfold AI, Avenda Health’s AI-powered cancer mapping technology, was a better predictor of focal therapy success than other factors, including the size or grade of the tumor.
In the study, FDA-cleared Unfold AI technology was retrospectively applied to 118 previously untreated men undergoing hemi-gland cryotherapy for prostate cancer ablation. MRI-guided biopsies, both targeted and systematic, were performed before cryotherapy and six months after cryotherapy to assess treatment outcomes. The study found that the baseline Gleason Grade Group (GG), which categorizes the aggressiveness of prostate cancer (GG1 indicating low-grade and GG5 high-grade), consisted of 66.8% GG2, 28% GG3, and 5.3% GG4. Treatment success, defined as the absence of >GG2 on post-cryotherapy MRI-guided biopsies, was achieved in 77.7% of cases and was not found to be correlated with baseline GG.
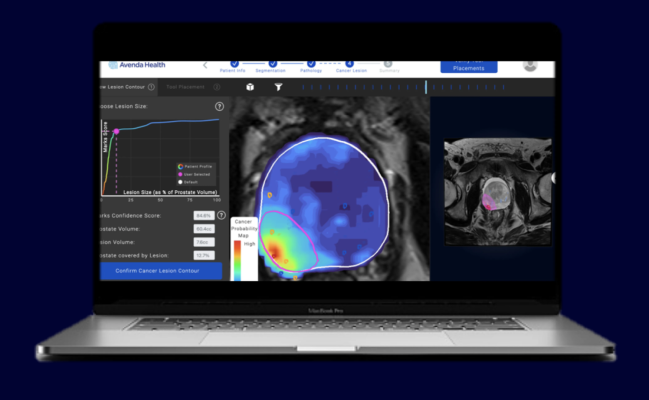
The study found that Unfold AI’s patient-specific encapsulation confidence score (ECS), which is generated based on multiple patient data points, including MRI scans, biopsy results, PSA data and Gleason scores, is critical for predicting treatment success. While factors like cancer core length, the percentage of Gleason pattern 4, and ECS score were predictive of treatment success, only an ECS score greater than or equal to 0.7 demonstrated significant predictive value, with a success rate of 70% (confidence interval: 61-79%) and 68% (confidence interval: 51-85%), respectively. These findings emphasize the importance of Unfold AI’s assessment of tumor margins in predicting treatment outcomes, surpassing the predictive capability of conventional parameters.
The study’s findings hold important implications for clinical practice, suggesting that Unfold AI’s ECS score could revolutionize treatment decision-making for prostate cancer patients. By increasing confidence in predicting tumor margins, Unfold AI empowers physicians to tailor interventions more effectively to individual patient needs, potentially improving treatment outcomes and quality of life.
“Unfold AI’s ability to identify tumor margins and provide the ECS will improve treatment recommendations and allow for less-invasive interventions,” said Dr. Wayne Brisbane. “This more comprehensive approach enhances our ability to predict treatment outcomes and tailor interventions effectively to individual patient needs.”
“These results demonstrate a marked change in how physicians will be able to diagnose and recommend treatment for prostate cancer patients,” said Avenda Health CEO Shyam Natarajan, PhD. “By increasing the confidence in which we can predict a tumor’s margins, patients and their doctors will have increased certainty that their entire tumor is treated and with the appropriate intervention in correlation to the severity of their case.”
For more information, visit avendahealth.com


 March 06, 2026
March 06, 2026 









