July 17, 2015 - Molecular breast imaging (MBI/BSGI) has been proven to be a highly sensitive imaging technique for the diagnosis of invasive lobular carcinoma (ILC), a position supported by a recent publication in the Journal of the American Medical Association Surgery. Physicians at the Legacy Cancer Institute in Portland, Oregon, reported a sensitivity of 88.9 percent for MBI/BSGI in diagnosing ILC, a difficult-to-diagnose breast cancer.
This study was a retrospective review of a prospectively maintained imaging registry at the institution in the course of six years. The analysis was conducted with almost 700 patients who underwent the BSGI procedure, which to date makes it the largest pool of data on molecular imaging of ILCs. The results show that BSGI has a slightly higher sensitivity for the detection of ILCs than for the detection of invasive ductal carcinomas (IDCs), while the specificities for both diseases are comparable. BSGI was also reported to detect additional cancers in patients with ILC. BSGI was found to have advantages over magnetic resonance imaging (MRI), as it is easier to interpret, better tolerated by patients and much less costly. The group of physicians concluded that BSGI has high sensitivity and specificity and is an excellent adjunctive imaging tool for patients with newly diagnosed ILCs.
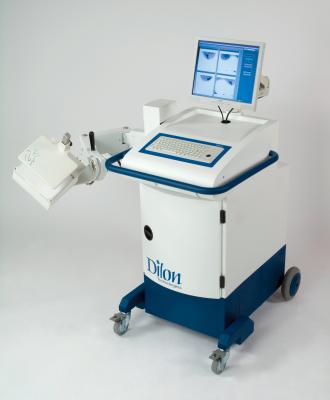
The MBI for the study was conducted using a Dilon molecular imaging system, a high-resolution, small field-of-view gamma camera, optimized to perform MBI. With MBI, the patient receives a pharmaceutical tracing agent that is absorbed by all the cells in the body. Due to their increased rate of metabolic activity, cancerous cells in the breast absorb a greater amount of the tracing agent than the normal surrounding tissue and generally appear as "hot spots" on the MBI image.
For more information: www.dilon.com


 February 18, 2026
February 18, 2026 









