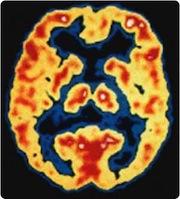
October 19, 2011 – The development of Alzheimer’s disease drugs and early detection has been hindered for years because no imaging agents or diagnostic tests existed. The only definitive way to diagnosis the disease was through cadaver brain biopsy samples. However, new radiotracers have been developed to illuminate the beta amyloid plaques that accumulate in specific areas of the brain in Alzheimer’s patients.
Using positron emission tomography (PET) and the radiotracer 11-C Pittsburgh Compound B (PIB), Roche said treatment with its drug candidate monoclonal antibody gantenerumab showed amyloid decrease in the brain of Alzheimer patients. This is the objective of the SCarlet RoAD trial, which will investigate the efficacy and safety of gantenerumab in patients in the early or prodromal stage of Alzheimer's disease.
With early or prodromal Alzheimer's disease, a person's memory loss is worse than can be expected by the normal aging process alone; even so, their ability to get on with daily activities is not affected to such an extent that they would be diagnosed with dementia.
The study used gantenerumab at two different doses or placebo. Brain amyloid was measured in 16 patients with mild to moderate Alzheimer's disease using PET and PIB. Alzheimer's disease brain slices from an independent patient sample were incubated with gantenerumab at increasing concentrations and with human microglia in an ex vivo phagocytosis assay.
The next step is investigate whether removal of brain amyloid translates into clinical benefit for patients at doses of gantenerumab that reduce brain amyloid and are well tolerated, with a favorable safety profile.
The study, "Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab," is published in the October issue of Archives in Neurology.
It is the first time clinical data has been published for gantenerumab, an investigational compound with a mechanism of action targeted at the early stages of Alzheimer's.
Results from Phase I clinical trials and ex vivo studies demonstrated that gantenerumab treatment results in a dose-dependent reduction of brain amyloid, possibly through phagocytosis via brain microglial cells. Amyloid load increased in patients receiving placebo treatment.
"These results and especially the rapidity of the effects observed on amyloid removal are very encouraging and pave the way for the development of a novel treatment for Alzheimer's disease," said Luca Santarelli, global head of Roche neuroscience disease translational area. "Our approach is to utilize biomarkers to diagnose and treat the disease at a very early stage before significant damage to the brain has occurred."
Gantenerumab is an investigational fully human anti-amyloid beta monoclonal antibody designed to bind to amyloid plaques in the brain and remove them. It is hoped this approach will slow progression of the disease, an outcome that cannot be achieved with currently approved treatments.
"Our objective was not only to demonstrate the effects of gantenerumab on brain amyloid, but also to start elucidating its mechanism of action," added Santarelli, "this is extremely important to fully understand the compound's therapeutic potential for Alzheimer's disease."
Since amyloid accumulates in patients' brains about 15 years prior to the onset of dementia, future clinical studies with gantenerumab will focus on Alzheimer's in the early or prodromal phase. It is hoped early diagnosis and intervention, before significant damage to nerve cells has occurred, will offer optimal benefit to patients.
For more information: www.roche.com


 January 28, 2026
January 28, 2026