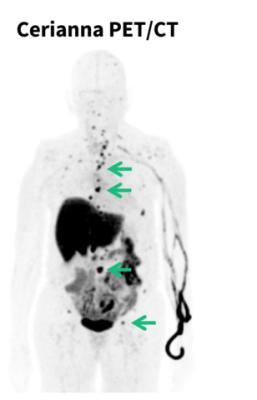
May 30, 2025 — GE HealthCare recently announced that the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for clinicians now recommend considering fluoroestradiol (FES) positron emission tomography (PET) for systemic staging in patients with recurrent or metastatic lobular breast cancer.
GE HealthCare’s Cerianna (fluoroestradiol F18) injection, available in the United States, is the only FDA-approved imaging agent for the detection of estrogen receptor positive (ER+) breast cancer metastases, including lobular breast cancer. The updated NCCN Guidelines expand the recommendation for the use of FES PET imaging in ER+ disease. In 2023, FES PET was included in the NCCN Guidelines for systemic staging of recurrent/stage IV ER+ breast cancer.
According to the Breast Cancer Research Foundation, 95% of invasive lobular cancer (ILC) tumors are ER+.[i] ILC is the second most common type of breast cancer in the U.S., accounting for 10-15% of all breast cancers and an estimated 43,000 new cases each year.[ii] [iii] [iv]
Lobular tumors typically do not form lumps which makes the cancer harder to detect with self-exams, mammography, ultrasound and magnetic resonance imaging (MRI). ILC can also recur more than a decade[v] after initial diagnosis and metastasize to unusual places such as the bones, brain, liver, lungs, gynecological organs, and others.[vi][vii] Once recurrent or metastatic lobular breast cancer is suspected, the new guidelines recommend considering FES PET imaging of the whole body to assess whether ER+ lobular tumors are present.
“Lobular cancers are often missed during routine screening, which can result in larger, more advanced tumors when they are finally detected and diagnosed,” said Jit Saini, MD, Chief Medical Officer of the Pharmaceutical Diagnostics (PDx) division of GE HealthCare. “This NCCN Guidelines update is significant because it will give more oncologists the confidence to use Cerianna PET imaging for patients with lobular breast cancer. It may also facilitate broader insurance coverage so more patients with this common, but hard-to-detect cancer will have an opportunity to receive a comprehensive diagnosis that accelerates clinical decisions and early intervention.”
Cerianna is a molecular imaging agent indicated for use with PET imaging for the detection of ER+ lesions as an adjunct to biopsy in patients with known or suspected recurrent or metastatic breast cancer. Cerianna works by binding to functional ER lesions and then the whole body is imaged by PET scan to help doctors assess and treat lobular breast cancer appropriately. With its diffuse growth patterns, detection and biopsy of ILC can be more difficult. Cerianna provides an alternative method to assess ER status across the whole body.
The recommendation that FES PET may be appropriate in lobular histology was established by an expert working group convened by the Society of Nuclear Medicine and Molecular Imaging (SNMMI) and included breast care specialists from the SNMMI, Lobular Breast Cancer Alliance, American College of Nuclear Medicine and Korean Society of Nuclear Medicine. The SNMMI is a nonprofit organization that promotes the science, technology and practical application of nuclear medicine and molecular imaging.
The National Comprehensive Cancer Network (NCCN) is a not-for-profit alliance of 33 leading U.S. cancer centers devoted to patient care, research, and education. The NCCN Guidelines are the recognized standard for clinical direction and policy in cancer care and the most thorough and frequently updated clinical practice guidelines available in any area of medicine.
Learn more about Cerianna here: https://www.gehealthcare.com/products/molecular-imaging-agents/cerianna.
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
[i] Rubio M, et al. Invasive lobular carcinoma: symptoms, treatment, research. Breast Cancer Res. 2025 May 20. Available from: https://www.bcrf.org/about-breast-cancer/invasive-lobular-carcinoma/.
[ii] McCart Reed AE, et al. Invasive lobular carcinoma of the breast: the increasing importance of this special subtype. Breast Cancer Res. 2021 Jan 7;23(1):6. PMID: 33413533
[iii] Ciriello G, et al. Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell. 2015 Oct 8;163(2):506-19. PMID: 26451490
[iv] 2021 Projected Incidence from ACS Surveillance Research (Source: SEER data)
[v] Pestalozzi BC, et al. International Breast Cancer Study Group. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol. 2008 Jun 20;26(18):3006-14. PMID: 18458044
[vi] Franzoi MA, et al. Leptomeningeal carcinomatosis in patients with breast cancer. Crit Rev Oncol Hematol. 2019 Mar;135:85-94. PMID: 30819451
[vii] Blohmer M, et al. Patient treatment and outcome after breast cancer orbital and periorbital metastases: a comprehensive case series including analysis of lobular versus ductal tumor histology. Breast Cancer Res. 2020 Jun 26;22(1):70. PMID: 32586354


 December 03, 2025
December 03, 2025 








