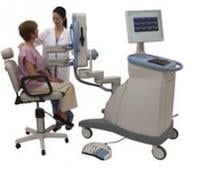
October 21, 2011 — The National Institute of Cancer (INCan) is the first to offer positron emission mammography (PEM) to the residents of Mexico. PEM is the breast application of the Naviscan high-resolution positron emission tomography (PET) scanner that shows the location as well as the metabolic phase of a lesion. It is the only U.S. Food and Drug Administration (FDA)-cleared 3-D molecular breast imaging (MBI) device on the market.
The scanner is compact, mobile and easy-to-use for precisely characterizing breast cancer, enhancing surgical planning, monitoring patient response to chemotherapy and evaluating suspected recurrence. It is designed to show the metabolic phase of a lesion, allowing for early breast cancer diagnosis and assisting physicians in making optimal care decisions. It provides the ability to distinguish between benign and malignant lesions - what researchers term “specificity.”
“With the arrival of PEM to the National Institute of Cancer in Mexico, we are offering our patients a diagnostic tool that will allow us to more accurately localize lesions as well as characterize them at a much earlier stage, with which we expect to reduce the harm that cancer causes our population,” said Enrique Estrada-Lobato, M.D., chairman of the nuclear medicine and molecular imaging department of the National Institute of Cancer in Mexico.
For more information: www.naviscan.com


 January 27, 2026
January 27, 2026