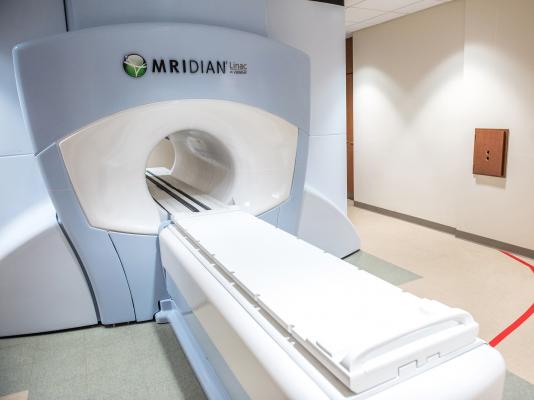
March 21, 2018 — ViewRay Inc. recently announced that the company received Shonin approval from the Japanese Ministry of Health, Labor and Welfare (MHLW) to market the MRIdian Linac System featuring linear accelerator (Linac) delivery. The MRIdian cobalt system received Shonin approval in August 2016.
MRIdian is the first and only magnetic resonance imaging (MRI)-guided radiation therapy system to receive Shonin approval in Japan, the world's third largest market for radiation oncology. One MRIdian cobalt system is currently treating in Tokyo at the National Cancer Center (NCC) and one is under installation at the private Edogawa Hospital.
The MRIdian Linac System combines MR imaging with linear accelerator radiation delivery so clinicians can visualize the tumor and nearby soft tissue and organs in real-time. Like the MRIdian cobalt system, MRIdian Linac enables daily on-table adaptive radiotherapy and real-time tracking to adjust radiation beam delivery dynamically for subtle anatomical changes that may occur, both during treatment delivery and throughout the course of treatment. Combined, these capabilities provide the potential for clinicians to improve targeting precision and thus deliver higher radiation doses.
ViewRay is represented in Japan by ITOCHU Corp., one of the three largest general trading companies in Japan.
For more information: www.viewray.com


 February 13, 2026
February 13, 2026 









