August 15, 2013 — Mevion Medical Systems Inc. has secured $55 million to accelerate the deployment of its revolutionary Mevion S250 Proton Therapy System. The funds were raised from existing equity investors, including Caxton Heath Life Sciences, ProQuest Investments, Venrock and CHL Medical Partners, as well as from debt financing provided by Life Sciences Alternative Funding LLC (LSAF).
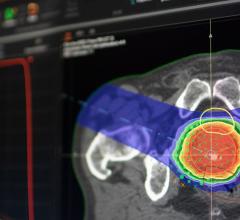
Proton particles have the intrinsic ability to deliver a more highly targeted treatment for the management of cancerous tumors and lesions as compared to traditional X-ray radiation therapy. This ability can provide less collateral damage to surrounding healthy tissue, making proton therapy a valuable treatment option for pediatric cancers and high-risk target locations. However, proton therapy has been limited to only a select few institutions because of the high-cost, large space requirements and operational complexities of legacy proton systems. The Mevion S250 is a proton therapy system that has changed this paradigm, delivering the same precise, non-invasive treatment capabilities and advantages of conventional systems but with a significantly reduced size, improved reliability, efficient clinical workflow and lower implementation cost. With a footprint similar to a modern X-ray radiation therapy device, the Mevion S250 brings accessibility, affordability and practicality to proton therapy.
“Mevion was founded to provide cancer centers and patients around the world with cutting-edge proton therapy technology on a scale, size and cost that is accessible and practical for today’s healthcare landscape,” said Joseph K. Jachinowski, chief executive officer of Mevion Medical Systems. “These additional funds will support the growth of our business operations in the U.S. and internationally.”
The Mevion S250 proton therapy system is the only single-room proton therapy system that is cleared by the U.S. Food and Drug Administration (FDA) for clinical use. The first Mevion S250 is installed at the S. Lee Kling Center for Proton Therapy at Barnes Jewish Hospital at Washington University Medical Center in St. Louis, Mo., and is currently undergoing clinical acceptance and commissioning in preparation for patient treatment later this year. With five centers under installation and construction and more than a dozen under planning, Mevion has established itself as a leading proton therapy supplier worldwide
For more information: www.mevion.com


 May 06, 2024
May 06, 2024