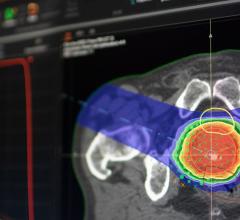
September 12, 2014 — Mevion Medical Systems introduced Hyperscan for the Mevion S250 modular single-room proton therapy system. Benefiting from direct and efficient proton beam generation, Hyperscan’s patented technology allows radiation to be precisely delivered using a sharp beam of radiation, with the tumor being volumetrically scanned in a matter of seconds. Current pencil beam delivery systems are known to be sensitive to motion, which can result in higher treatment uncertainties. Hyperscan’s fast beam delivery is much less sensitive to patient and tumor motions, thus delivering a more robust treatment.
“Hyperscan enables treatment with fine precision at tremendous speed and with uncompromised beam quality,” said Joseph K. Jachinowski, president and CEO of Mevion Medical Systems. “This new technological advance will help providers deliver the best possible cancer care to the patients who need it most.”
Hyperscan is debuting at the 56th annual meeting of the American Society for Radiation Oncology (ASTRO), being held Sept. 14-16 in San Francisco. It is built on the Mevion S250 proton therapy platform that has been operating clinically since December 2013. The Hyperscan system features rapid volumetric and layer rescanning, a streamlined image-based clinical workflow, sub-millimeter position tracking and beam gating connectivity.
Simultaneously, Mevion is announcing it has reached an agreement with a financial services provider to offer financing to customers interested in purchasing the Mevion S250. This will provide Mevion’s customers with an alternative to traditional financing structures, should they desire it.
“The introduction of Hyperscan technology and the availability of alternative financing is a demonstration of Mevion’s continued commitment to improving the worldwide availability and clinical use of proton therapy,” said Jachinowski. “Mevion is dedicated to removing the obstacles of size, complexity and cost that exist with conventional proton therapy systems and making proton therapy as accessible as possible to the cancer patients who need it.”
The U.S. Food and Drug Administration (FDA) has not cleared Hyperscan for clinical use.
For more information: www.mevion.com



 May 06, 2024
May 06, 2024