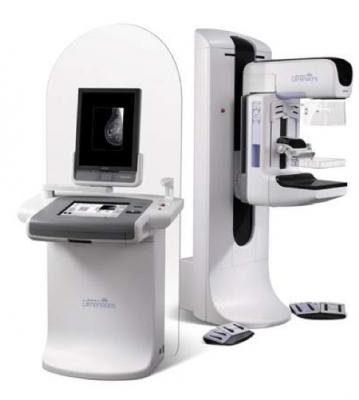
December 12, 2018 — A Massachusetts Superior Court granted a preliminary injunction in lawsuits by Hologic against Chinese X-ray equipment provider Direct Digital Imaging Technology (Beijing) Inc. (DDIT) and a former Hologic employee, Lawrence Ibbetson, for alleged misappropriation of trade secrets.
Hologic alleges that DDIT and Ibbetson misappropriated trade secrets for selenium coating technology used in the detectors within Hologic's Selenia Dimensions and 3Dimensions mammography systems. In 2016, Hologic filed suit against DDIT, alleging breach of contract for failure to pay for general radiography panels treated with Hologic’s proprietary coatings. In 2018, the complaint was amended to include trade secret misappropriation claims.
As part of the preliminary injunction, the court ruled that Hologic is likely to succeed in its trade secret claims. As a result, the court ordered the defendants to stop using or disclosing selenium coating specifications that Hologic alleges were wrongfully misappropriated. In addition, the defendants may not develop an alternative selenium coating unless certain conditions are met, including that the development be conducted under the watch of an independent monitor. The preliminary injunction is effective through the conclusion of a trial.
For more information: www.hologic.com


 February 18, 2026
February 18, 2026 









