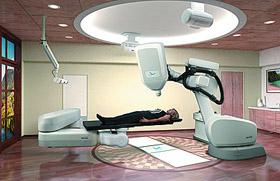
The Japanese Ministry of Health, Labor and Welfare (MHLW) gave the latest CyberKnife Radiosurgery the Shonin approval.
The CyberKnife G4 Robotic Radiosurgery Systems from Accuray Inc., will be available in Japan to treat tumors non-invasively anywhere in the body, including head and neck.
This regulatory approval dramatically expanded the types of Japanese patients that could be treated with radiosurgery to include those with cancers of the spine, lung, liver, pancreas and prostate.
The CyberKnife G4 System addresses the unique challenges of random and excessive target motion by using intelligent and adaptive image guidance, which minimizes dose to surrounding healthy tissue and critical structures, ultimately minimizing treatment complications and side effects.
The CyberKnife G4 System will provide Japanese patients with some of the latest advances in CyberKnife technology including: Motion management technology to correct for tumor motion including the Xsight Lung Tracking System, which eliminates the need for fiducials in many lung cases; Hardware and software enhancements that enable up to 50 percent reduction in treatment time; Customized treatment plans dictated by clinical requirements and objectives; and Flexibility to adapt the fractionation scheme to meet the unique needs of each patient simply and conveniently in routine clinical practice.
With 21 CyberKnife Systems installed throughout Japan, it is the second largest installed base of CyberKnife Systems after the United States.


 January 30, 2026
January 30, 2026 









