November 10, 2017 – Kubtec announced it will be showcasing new image analytics capabilities on its advanced tomosynthesis specimen imaging systems for breast cancer at the 2017 Radiological Society of North America (RSNA) conference, Nov. 26-Dec. 1 in Chicago.
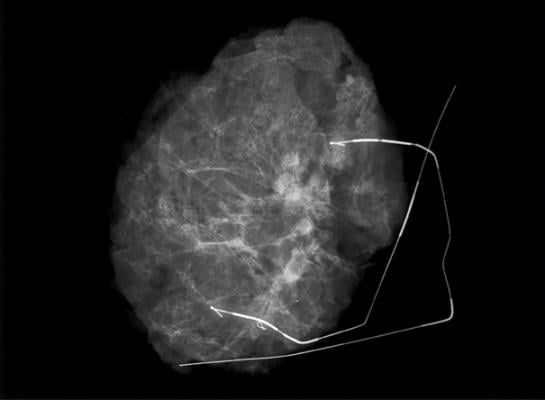
The company uses 3-D tomosynthesis, which creates images in 1mm digital slices to give radiologists, surgeons and pathologists an improved view of the anatomy of surgical specimens. This enables them to reduce the incidence of positive margins that often lead to repeat surgeries.
Kubtec’s new image analytics technology automatically highlights microcalcifications and other inclusions in the specimen, and identifies and displays their exact location within the specimen. Remote voice control enables medical staff to operate the instruments without breaking scrub.
Kubtec CEO Vikram Butani said the company is focused on bringing augmented intelligence to the intraoperative imaging space. He added that its patent-pending artificial intelligence (AI) program provides additional functionality that reduces errors and oversights, reduces surgical procedure times and enables improvements in patient outcomes.
For more information: www.kubtec.com


 March 06, 2026
March 06, 2026 









