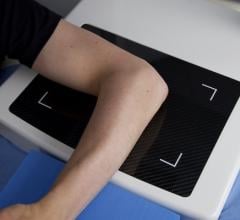
Konica Minolta Medical Imaging announces the availability of a 14” x 51” long-length cassette assembly for use with the Xpress CR and IQue CR systems. The 14” x 51” long-length cassette assemblies are designed for the digital capture of full-length leg and full-length spine of patients taller than 6’6”, with one single exposure.
“While our current 14” x 42” cassette assembly has been well accepted as it accommodates most of the population, the 14” x 51” cassette will offer radiology and orthopedic users the flexibility to accommodate exceptionally tall patients,” says Eunice K. Lin, Marketing and Product Planning Manager.
The long-length cassette houses three slightly overlapped CR plates to capture the entire length of the spine or leg on multiple CR plates simultaneously. These plates are then inserted onto cassettes of the same size for scanning by the CR reader. The Stitching software on the Xpress CR and IQue CR Control Stations then automatically and seamlessly joins these individual images as one composite image. This enables radiologists and orthopedic surgeons to easily view and measure the body parts for diagnosis or surgical planning.
A 14” x 51” wall-mount cassette holder is also available for holding the cassette. It has a built-in grid with 10:1 ratio, 178 line pair per inch and a focal distance of 60-90 inches. Konica Minolta will offer both 14” x 51” and 14” x 42” long-length cassettes, providing customers the right solution based on their clinical applications, patient demographics and space requirements.


 January 22, 2026
January 22, 2026