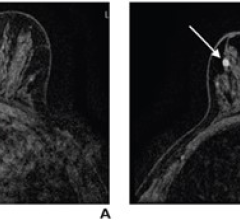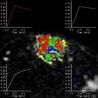
kinetic curves of breast
August 24, 2009 - Breast MRI allows physicians to evaluate suspicious lesions using a variety of variables and computer-aided kinetic information can help significantly in distinguishing benign from malignant suspicious breast lesions on MRI, according to a study published in the September issue of the American Journal of Roentgenology (AJR). In the study, performed at the University of Washington Medical Center, researchers analyzed and compared the computer-aided evaluation variables of 125 suspicious breast lesions. Three different kinetic curves (washout, plateau and persistent), were compared along with lesion morphology (size and shape). Researchers wanted to clarify which, of the many variables that reflect kinetics, were most predictive of malignancy. The investigators found overlap in kinetic patterns across benign and malignant lesions, but did determine that the "most suspicious" curve type, washout, was useful in separating benign from malignant lesions. According to Constance Lehman, MD, lead author of the study, of the lesions with the most suspicious curve type (any washout), 45.7 percent were malignant compared with 20.0 percent with plateau and 13.3 percent with entirely persistent enhancement. "We continue to study the specific features on MRI most predictive of breast cancer. We know that the morphology of the lesion is extremely important, but our study also supports the use of kinetic features in lesion assessment. The "most suspicious" curve, washout, does seem to help distinguish benign from malignant lesions," said Dr. Lehman. For more information: www.arrs.org


 February 18, 2026
February 18, 2026