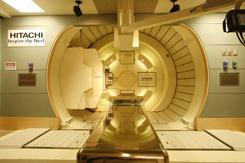
June 22, 2015 - Hitachi Ltd. announced that Johns Hopkins Medicine has selected Hitachi to provide its proton beam therapy (PBT) system at Sibley Memorial Hospital in Washington, D.C. This collaboration, which includes a 10-year maintenance service, marks the first multi-room PBT application in the nation's capital. This will be Hitachi's fifth PBT system in North America.
The next-generation system "Probeat", which comes with intensity modulated proton therapy (IMPT) and cone-beam computed tomography (CT), will have improved spot scanning capability in all three gantry-type treatment rooms, along with a fixed irradiation room dedicated to cancer research.
In December 2007, Hitachi was the first company in the U.S. to clear U.S. Food and Drug Administration (FDA) Premarket Notification Special 510(k) for the "Probeat" system with its spot scanning irradiation technology. The same spot scanning system has already been installed at Nagoya Proton Therapy Center and Hokkaido University in Japan. In fiscal year 2015 (ending March 2016), one of Hitachi's new PBT site is planning to start treatment of patients.
Unlike conventional scattering technology, spot scanning technology delivers narrow beams to the tumor and the complex tumor shape can be irradiated through repetitive beam delivery with quick position change. Spot scanning technology has been achieved by advancing the uniform quality beam extraction technology from the accelerator and beam control technology with high accuracy.
For more information: www.hitachi.com


 January 30, 2026
January 30, 2026 









