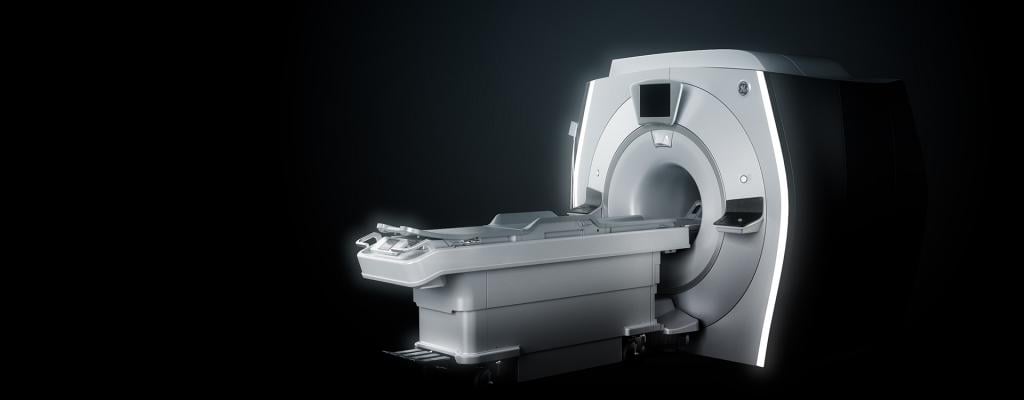
July 10, 2019 — GE Healthcare and Insightec announced U.S. Food and Drug Administration (FDA) approval and CE mark for Insightec’s Exablate Neuro compatible with the Signa Premier magnetic resonance imaging (MRI) system from GE Healthcare. The Exablate Neuro is a focused ultrasound platform for treating deep in the brain with no surgical incisions. MR imaging provides a comprehensive anatomical survey of the treatment area, patient-specific planning and real-time thermal monitoring throughout the treatment.
Exablate Neuro has FDA approval for the treatment of medication-refractory essential tremor and tremor-dominant Parkinson's disease. It has CE mark for the treatment of essential tremor, tremor-dominant Parkinson's disease-unilateral and neuropathic pain.
For more information: www.insightec.com, www.gehealthcare.com


 February 13, 2026
February 13, 2026 









