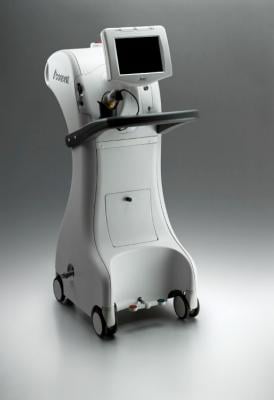
iCAD's Xoft Axxent electronic brachytherapy (eBx) system
September 11, 2014 — iCAD Inc. will debut the company's latest technological advances and present updates on clinical data and patient outcomes using the Xoft Axxent electronic brachytherapy (eBx) system in the treatment of cancer during the upcoming American Society for Therapeutic Radiology and Oncology (ASTRO) meeting, Sept. 14-17.
Throughout the meeting iCAD will also showcase its expanded and comprehensive software and service offerings following the recent acquisitions of DermEbx and Radion, and will highlight innovations in technology with live product demonstrations of its Xoft system at booth #1938.
"This is a very exciting year for iCAD as we have significantly expanded our position as the industry's leading comprehensive solution provider in eBx. We look forward to outlining the elements of our new solutions and services and to giving customers a hands-on experience with our recent advances to the technology platforms, including the debut of our Axxent SPX controller, the latest extension of the Xoft system product line," said Ken Ferry, president and CEO of iCAD. "With our progress this year, iCAD is uniquely positioned to provide significant technology and service advantages to our valued customers and their patients."
"We continue to be encouraged by the positive data presented on the Xoft system for the treatment of non-melanoma skin cancer, as it further demonstrates the system's distinct clinical and cosmetic advantages," added Ferry.
During ASTRO iCAD will debut the new Axxent SPX controller, the latest extension of the Axxent product line. The Axxent SPX controller features a streamlined design to support enhanced mobility and flexibility for delivery of electronic brachytherapy treatment in a wider range of healthcare settings, including smaller treatment rooms. The controller also features new software and an enhanced barcode scanning function that offers significant workflow benefits and improved technology support for HIPAA compliance. These features are also now available on the multiplatform Axxent MPX controller, which remains commercially available as the company's platform product approved for use in all indications including breast intraoperative radiation therapy (IORT), breast accelerated partial breast irradiation (APBI), skin, endometrial and cervical cancer treatments.
"Electronic brachytherapy allows radiation oncologists to expand the reach of HDR [high-dose-rate] brachytherapy beyond conventional shielded vaults. It inherently offers safety for patients and personnel, target flexibility for challenging anatomic areas and fine radiation dose control. The new streamlined design may be easier to integrate into smaller spaces in many clinical facilities," said Rakesh Patel, M.D., medical director at the Targeted Radiation Institutes in the East and South Bay Areas in California.
For more information: www.xoftinc.com, www.icadmed.com


 January 30, 2026
January 30, 2026 









