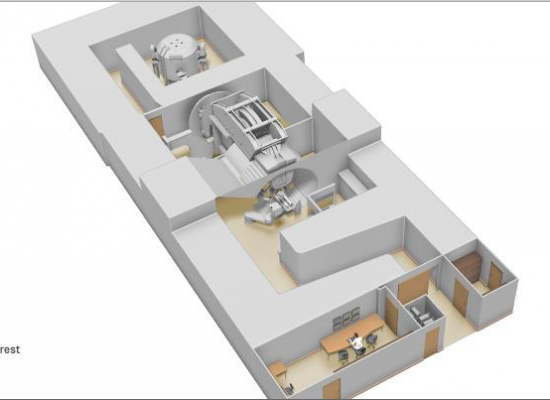
April 28, 2017 — IBA announced the delivery and rigging of three Proteus One treatment rooms in less than a week at centers in three different countries in early April. The centers include The Rutherford Cancer Centre, South Wales, United Kingdom); Cyclhad (Cyclotron for Hadron Therapy) located in Caen, France; and Hokkaido Ohno Memorial Hospital located in Sapporo, Japan.
All three centers will be equipped with IBA’s compact Proteus One solution, comprising the latest technology of image guided, intensity modulated proton therapy. Being able to ship and rig three compact proton therapy solutions in less than a week is a world first, according to IBA.
For more information: www.iba-worldwide.com


 January 30, 2026
January 30, 2026 









