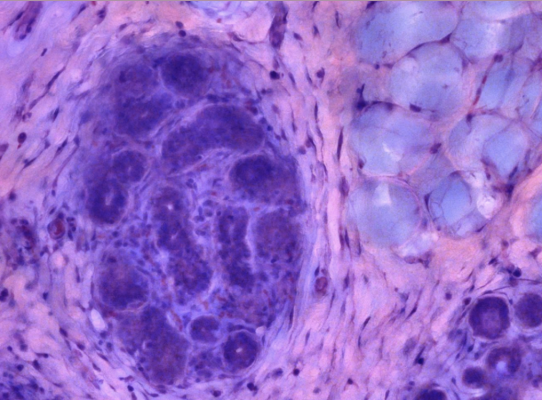
Benign breast in collagenous stroma with adipose stroma (top right). Lobular structure, capillaries, and surrounding fibroblast are easily recognized. Image courtesy of Histolix
October 18, 2021 — Histolix, a leading developer of direct-to-digital read pathology solutions, announced it has launched a groundbreaking clinical trial to prove equivalency of its digital technology to current gold standard pathology testing. The company’s direct-to-digital read solution is designed to reduce today’s labor-intensive slide-based pathology preparation and reads, which can take in excess of eight hours to produce, to less than 10 minutes. Leading pathology experts believe the Histolix technology could provide a radical new approach to the preparation of biopsy specimens.
“The Histolix technology heralds a radically new approach to the preparation of the images required by pathologists to interpret biopsy specimens,” said Allen Gown, M.D., former professor at the University of Washington Medical Center and founder of PhenoPath Laboratories. “Since its origins in the 19th century, pathology has been constrained by a laborious and time-consuming process of fixation, dehydration, paraffinization, rehydration and staining requiring skilled technologists and expensive equipment. Histolix technology promises to dramatically reduce the time required to prepare these images and simultaneously permits a direct-to-digital pathologic evaluation framework, permitting pathology to emulate radiology in rapid evaluation and dissemination of biopsy findings.”
Gown further believes Histolix generates digital images suitable for pathologic evaluation accessible to a wide range of users, including satellite lab facilities, veterinary laboratories and resource-constrained laboratories all over the world.
Histolix plans to release the findings of its initial clinical validation study comparing direct-to-digital technology diagnosis versus standard FFPE tissue section H&E slides by the end of the year. The comparison will show diagnostic accuracy of Histolix imaging in under 10-minute acquisition of over 20 tissue types. A follow-on trial in 2022 will include lumpectomy intra-operative margin assessment, targeting up to 30 percent of the re-surgical procedures that occur today in breast cancer and other surgeries due to limitations of today’s standard, frozen section techniques for tissue margin analysis.
“After decades of minimal change to the WSI market, it's exciting to see a technology such as Histolix impacting direct read of tissue at point-of-care and in the operational suites,” said Rob Royea, president and CEO of Histolix. “Histolix is proving the viability of this technology with expert third party pathologists reviewing and providing diagnosis for 100 surgical cases from both Histolix images and in standard glass slide whole slide images, in random order with a “washout” between reads. We are excited to partner with renowned pathologists from UC Davis who founded this technology and guide the clinical validation trials. It is also our honor to have Dr. Gown join us in this historic endeavor.”
The pathology market is immense with over 4.5M biopsies performed per year and over 1.2M surgeries accelerating at a six percent rate annually. The global cancer biopsy market is projected to reach $33B USD by 2027. Histolix direct-to-digital read from fresh tissue across multiple tissue types has the potential to replace a sizable percentage of current pathology processes while significantly decreasing headcount and tissue handling cost, along with the possible elimination of up to 30 percent of non-necessary repeated surgical procedures.
“With today's embedded cost of whole slide imaging including redundant processes, transportation and delayed diagnosis, Histolix could significantly impact cost reduction, enable short term treatment and reduce personal trauma due to delayed diagnosis,” concluded Royea. “Moreover, Histolix technology will enable expert telepathology and AI molecular level assessment globally ensuring peace of mind for diagnosticians and patients alike. We look forward to publicizing our clinical trials in the near future.”
For more information: www.histolixinc.com


 February 04, 2026
February 04, 2026 









