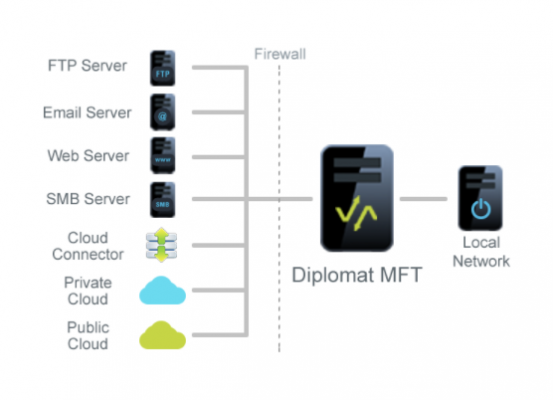
May 4, 2020 — Coviant Software, a provider of managed file transfer software that provides an easy way to automate, secure, manage, and audit file transfers while maintaining the highest levels of security and compliance, has extended its Diplomat MFT product to support the Google Cloud Healthcare API. This move follows quickly after the announcement by Google that the Cloud Healthcare API is now generally available, making Coviant Software the first and only MFT vendor to fully support Google Cloud Healthcare API for data exchange.
The Google Cloud Healthcare API provides a suite of capabilities designed to enable standardized data exchanges between healthcare applications and solutions built on Google Cloud. It provides support for FHIR, DICOM, and HL7 data integration, and is fully HIPAA compliant. Once stored in data sets on the Google Cloud Platform, the healthcare data can be de-identified for privacy, and can be easily integrated into data processing, analytics, and AI on the platform.
Coviant Software Diplomat MFT provides automated, secure file transfers from any endpoint, to any endpoint. "Diplomat MFT already supports easily transferring data to Google Cloud Storage," said Greg Hoffer, CEO of Coviant Software. "When we learned that the Google Cloud Healthcare API plays an important role in the management, analytics, and exchange of this important and sensitive data, we quickly extended our solution to make integration into the Google Cloud Healthcare API as simple as ticking a box and filling in a few fields. Now healthcare customers can leverage the cost savings and security of Google Cloud storage and easily take advantage of the power and flexibility of Google Cloud Healthcare API."
For more information: www.coviantsoftware.com


 February 17, 2026
February 17, 2026 









