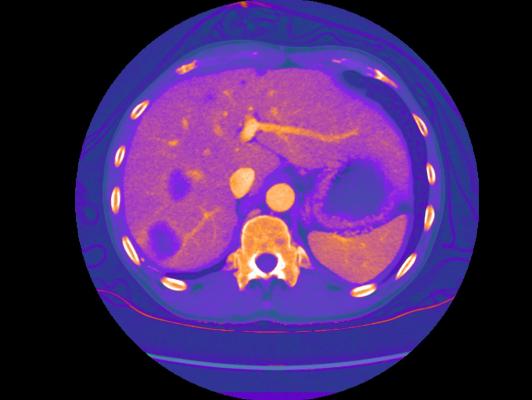
February 15, 2017 — GE Healthcare announced U.S. Food and Drug Administration (FDA) 510(k) clearance of GSI Xtream on Revolution CT (computed tomography). The company also announced the first series of clinical images and first installations at Duke University Medical Center (USA), Robarts Research Institute (Canada) and The First Affiliated Hospital of Dalian Medical University (China).
GSI Xtream on Revolution CT is the first volume spectral CT technology designed to improve small lesion detection, tissue characterization and metal artifact reduction, with a simplified workflow.
From setup to post processing, GE said GSI Xtream is as intuitive as a single energy exam. GSI Assist and Clinical ID help standardize and automate protocol selection with images directly transferred to picture archiving and communication systems (PACS) and/or automated workflow (AW). These improvements, combined with native GSI reconstruction, deliver spectral CT workflow that is twice as fast compared to the Revolution HD.
Spectral CT allows clinicians to go beyond anatomy visualization to tissue characterization, allowing for differentiation between similar HU densities such as cysts and enhancing lesions. With a contrast-to-noise ratio 60 percent higher at 120 kV than single energy CT, monochromatic images improve lesion detection and enhance contrast. Bhavik Patel, M.D., assistant professor of radiology, Duke Medical Center, said “By using GSI, we may be able to characterize a lesion for diagnosis from one exam, which could generate cost savings and add value – both clinically for the patient and economically for the health system.”
GSI Xtream enhances Revolution CT’s platform with wide collimation and 50 cm field-of-view (FOV) to deliver the only volume spectral CT. Enabled by ultrafast kV switching, clinicians can scan more challenging patients and reduce the effects of motion. The Gemstone Clarity detector combined with ASiR-V delivers dose neutral exams for patients of any size.
For more information: www.gehealthcare.com


 February 09, 2026
February 09, 2026 









