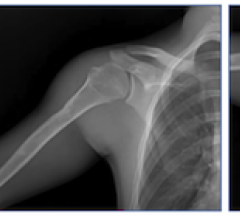
October 26, 2016 — Based on its recent analysis of the computerized tomography (CT) market for extremity imaging, Frost & Sullivan recognizes Carestream Health with the 2016 North American Frost & Sullivan Award for New Product Innovation. Carestream's U.S. Food and Drug Administration (FDA)-approved cone beam computerized tomography (CBCT) device, the OnSight 3-D Extremity System, is proving to be a game-changer with its ergonomic design and unparalleled features, according to Frost & Sullivan. Compared to multi-detector computerized tomography (MDCT) systems, this device delivers lower dosage and provides the ability to capture weight-bearing images.
The system’s intuitive design is distinct from conventional doughnut-shaped CT scanners. It consists of a wide door opening that provides easy, step-in patient access. The large imaging bore accommodates heavier patients, while enabling a wide field-of-view image capture. To facilitate convenient equipment positioning, it has a dual-side positioning joystick. On the top of the device, two detachable patient-support handles enhance safety and comfort. Finally, a monitor attached to the system displays the patient’s demographic details and the imaging status for individualized diagnoses of extremities.
"Carestream’s OnSight 3-D Extremity System utilizes advanced interactive reconstruction techniques and generates exceptional image quality in both bone and soft tissue,” said Frost & Sullivan Research Analyst Karan Verma. "Similarly, weight-bearing applications are better evaluated using this system. By delivering volumetric reconstruction that has isotropic spatial resolution in all three directions, it bridges the gap between the market and customer needs."
The system produces 3-D images of high diagnostic value at the point of care to evaluate subtle or occult fractures and their healing. The device can improve the visibility of patient anatomy even in the presence of metal implants, which are a common cause of noise in CT images. It improves workflow and productivity due to its ability to perform both 2-D and 3-D scans on the same device.
Carestream’s device expedites patient scans as it has a fast equipment setup option with pre-programmed auto-positions. The system will typically not require a large shielded room or specialized electrical service, enhancing the ease and cost effectiveness of the installation.
For the OnSight 3-D Extremity System, the company has a dedicated team that provides technical assistance to medical professionals in hospitals and private clinics.
For more information: www.carestream.com


 March 03, 2026
March 03, 2026