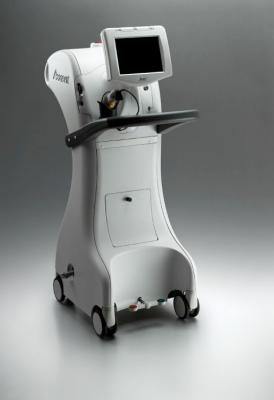
June 17, 2015 - iCAD Inc. announced the first Xoft Axxent Electronic Brachytherapy (eBx) System in Spain is now available at the Hospital Miguel Servet for the treatment of early-stage breast cancer, non-melanoma skin cancer and gynecological cancers. iCAD highlighted the Xoft System at the third European Society for Radiotherapy and Oncology (ESTRO) Forum in Barcelona, April 24-28.
Also during the ESTRO forum, Paulo Costa, M.D., radiation oncologist of Instituto CUF Porto, Breast Surgery Unit, Senhora da Hora, Matosinhos, Portugal, presented an update on his clinical research, which examines patients who underwent intraoperative radiation therapy (IORT) treatment using the Xoft System during breast conserving surgery between April 2012 and November 2014.
"With a median 18-month follow-up, clinical results continue to suggest that the Xoft System is safe with minimal side effects and low morbidity," said Costa. "As we continue to gather data, and patients and caregivers learn more about the benefits of IORT for the treatment of early-stage breast cancer, we are seeing increased patient demand for this radiation therapy option as a result of its much shorter course of treatment."
The Xoft System is an isotope-free radiation treatment cleared by the U.S. Food and Drug Administration and CE marked in the EU for use anywhere in the body, including for the treatment of early-stage breast cancer, gynecological cancers and non-melanoma skin cancer. It utilizes a proprietary miniaturized X-ray as the radiation source that delivers precise treatment directly to cancerous areas while sparing healthy tissue and organs. The Xoft System requires only minimal shielding and therefore does not require room redesign or construction investment. Minimal shielding also allows medical personnel to remain in the room with the patient during treatment. The mobility of the Xoft System makes it easy to treat patients at multiple locations and to easily store the system when not in use.
For more information: www.icadmed.com


 February 18, 2026
February 18, 2026 









