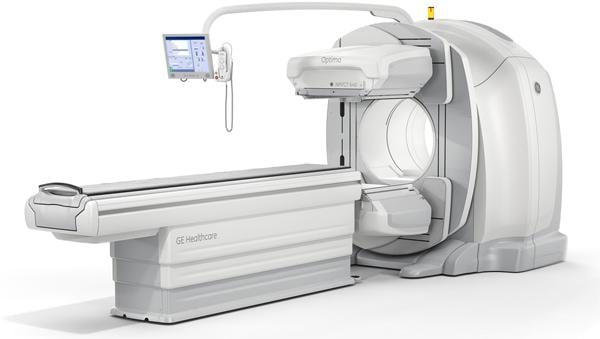
March 13, 2013 — GE Healthcare announced last month the first global commercial installation of its Optima NM/CT 640 technology, a new performance SPECT/CT (single-photon emission computed tomography/computed tomography) system that helps physicians balance high image quality with low patient radiation dose and efficiency. The installation is at Texas Health Harris Methodist Hospital Fort Worth in Fort Worth, Texas.
The Optima NM/CT 640 enables clinicians to focus on key needs in diagnostic imaging, including high-quality visuals, low radiation dose and short exam times for patients. The Optima NM/CT 640 allows for hybrid imaging rather than standalone CT use, integrating the latest generation general purpose camera with a newly developed four-slice CT. Available in 2.5 mm and 5 mm slice thicknesses, the CT technology is designed to optimize dose and resolution required for particular procedures, offering clinicians high-quality images with routinely low CT dose at 1-2 mSv for a 40 cm abdomen CT scan.
The Optima NM/CT 640 requires minimal renovation and installation costs and can be fully upgraded for future use, expanding its clinical applications as well as offering the potential for research capabilities. When used with the Xeleris workstation, Optima NM/CT allows clinicians to potentially reduce time or injected patient dose by up to 50 percent in most scanning procedures while maintaining image quality.
“We are dedicated to pushing nuclear medicine to its full potential and developing equipment that helps customers address the challenges they are confronted with every day — high image quality, low dose and short exam times,” said Nathan Hermony, general manager, nuclear medicine, GE Healthcare. “We are pleased to collaborate with clinical leaders at Texas Health Harris Methodist Hospital Fort Worth on the first global installation of Optima NM/CT 640 technology as they continue to deliver diagnostic excellence and quality patient care.”
Texas Health Fort Worth is a 726-bed, Magnet-designated regional referral center with more than 4,000 employees, 200 volunteers and nearly 1,000 physicians practicing on the medical staff. The hospital’s services include cardiovascular services, high-risk and routine obstetrics and gynecology, orthopedics and sports medicine, neonatal intensive care, and trauma/emergency medicine. Texas Health Fort Worth is an affiliate of the faith-based, nonprofit Texas Health Resources system.
For more information: www.gehealthcare.com


 February 03, 2026
February 03, 2026 









