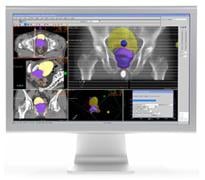
January 3, 2011 – The U.S. Food and Drug Administration (FDA) has given 510(k) clearance for software used for planning spot scanning. The XiO treatment planning software, from Elekta, is used with proton therapy, which helps clinicians deliver a highly targeted dose to a tumor.
Spot scanning is a proton therapy delivery method that involves constructing a highly conformal dose to the tumor by using thousands of small individual beamlets instead of a single large beam. This approach enables intensity modulated proton therapy (IMPT).
The software provides tools to facilitate rapid positioning of spots to construct the dose to the tumor, in addition to a proven dose calculation algorithm to optimize each beam.
"The completely automated optimization of the spot deliveries allows clinicians to produce treatment plans with a high degree of conformality around the target, therefore, minimizing the dose to adjacent healthy tissue," said Virgil Willcut, vice president of product management, physics and research for Elekta treatment planning. "Spot scanning especially is important for pediatric patients since it provides better normal tissue sparing and lower neutron doses than conventional proton planning, both of which reduce the chance of radiation induced side effects for a cohort of patients that are still developing and have long life expectancies."
The software has been in clinical use for nearly a decade.
For more information: www.elekta.com.


 February 11, 2026
February 11, 2026 









