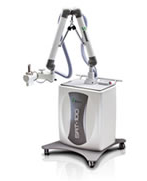
May 28, 2013 — Sensus Healthcare has received clearance from the U.S. Food and Drug Administration (FDA) to treat Keloids with the SRT-100. The SRT-100 is an alternative to surgery in treating non-melanoma skin cancer and now is approved to treat keloids caused by surgery or injury. Leading dermatologists and oncologists have found the SRT-100 superficial radiation therapy system to be a choice for their patients who are not good surgical candidates. Sensus Healthcare has reintroduced superficial radiotherapy (SRT) to the global clinical community as a viable, safe, and cost-effective modality to treat non-melanoma skin cancers and keloids in hospitals and private practice settings.
"Painful and potentially disfiguring keloid scars are very difficult to effectively treat by surgery or other means due to recurrence rates as high as 45 to 100 percent. Studies show that the use of adjunctive radiation therapy can dramatically reduce the rate of recurrences," said Mark S. Nestor, M.D., Ph.D., of the Center for Cosmetic Enhancement in Aventura, Fla.
"It is very fortunate that the FDA has approved the Sensus SRT-100, the first computerized, state-of-the-art, in-office SRT device for the treatment of recurrent keloid scars. Dermatologists now have a safe, effective in-office modality available to offer our keloid patients."
The SRT-100 has SharpBeam technology, where only the targeted lesion is being treated, while the surrounding and underlying healthy tissue is spared. The SRT-100 is FDA, CE, Health Canada and SFDA cleared and is manufactured in the United States.
For more information: www.sensushealthcare.com


 January 30, 2026
January 30, 2026 









