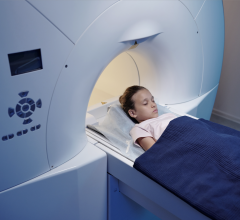
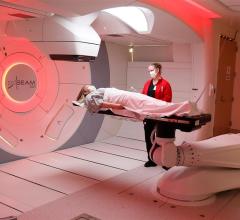
April 29, 2009 – The FDA cleared of a new generation of patient positioning system (PPS) developed by Ion Beam Applications S.A. (IBA) and ProCure Treatment Centers Inc. (ProCure), which is designed to improve proton therapy.
The basic configuration of the robotic PPS is a Selective Compliant Assembly Robot Arm (SCARA). It combines commercially available robotic sub-systems, which makes it cost-effective and easy to maintain, with custom designed arms to allow for the specific needs of patient positioning in proton therapy. A unique dual coupler system is attached at the end of the wrist of the robot arm that is used to attach different devices to the PPS, such as a patient table or chair, or a test phantom. The device employs reportedly safe robotic technology that meets the standards of the Robotics Industry Association (RIA).
Some specific design elements of the PPS compared to earlier generations include:
- An increase of the clinically usable area of the proton therapy treatment room. This larger work envelope also makes it suitable for image-guided proton therapy in combination with 3D imaging capability in the treatment room.
- Optimized movement speeds designed for safe and precise motion of the PPS. The range of speed and motion with the pitch-and-roll axes of the robot is restricted to reduce the sensation of movement when the patient is on the table.
- The ergonomics are said to be better, as there are no moving parts in the floor system that supports the device, which allows greater accessibility to the treatment area for patients and therapists
The new PPS is part of ProCure’s strategy to make proton therapy better and more accessible. This includes making the therapy more cost effective and creating a platform that will allow easy integration of future advanced image-guided proton treatments.
The first clinical installation will be at the ProCure Proton Therapy Center in Oklahoma City, scheduled to open this summer.
For more information: www.procure.com


 May 06, 2024
May 06, 2024