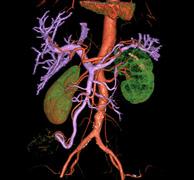
November 30, 2010 – The U.S. Food and Drug Administration (FDA) has given 510(k) clearance for a suite of clinical applications that is directly integrated into PACS and cardiovascular systems. FujiFilm Medical’s Synapse 3D is vendor neutral, multi-modality capable, and can be used with virtually any advanced imaging system.
It also provides access to advanced diagnostic tools without the need for separate workstations.
Several diagnostic clinical tools, ranging from 2-D, 3-D and 4-D viewing, to dynamic data, fusion (including PET, MR, CT and SPECT) and sector MPR (multi-planar reconstruction) are included.
Because it is integrated directly into the company's Synapse PACS and Synapse Cardiovascular, it is enterprise capable and available at any workstation where Synapse PACS is available. From the power jacket or “quick launch” mode, it can be readily accessed with just one click. This provides a more efficient workflow for clinicians by eliminating the need to access separate workstations with additional logons, while still maintaining security levels. It also automatically imports the advanced analysis back into the PACS and cardiovascular information (CVIS) systems, creating one common information database and image storage location.
“Synapse 3D has proven to be an incredibly valuable tool during our research, particularly its PET-MR fusion capabilities for diagnosing epilepsy and the utility of neurovascular calcium scoring for predicting stroke,” said Meng Law, M.D., director of neuroradiology, LAC and USC Healthcare Network. “The powerful and efficient post-processing capabilities have allowed us to see much more information to better support our diagnosis and patient care decisions, in some cases even enabling us to avoid surgeries. We’re all looking forward to the wide-scale rollout and the regular use of Synapse 3D by countless physicians and specialists across the LAC and USC Healthcare Network.”
For more information: www.fujimed.com


 February 20, 2026
February 20, 2026 









