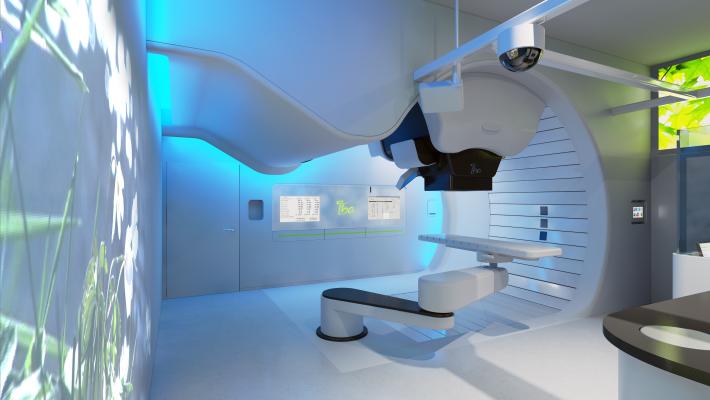
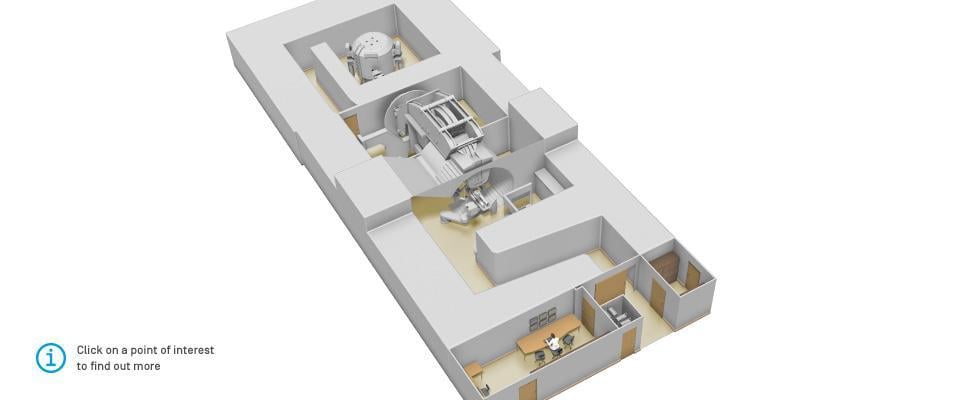
A room layout for the IBA ProteusOne compact proton therapy system.
August 18, 2016 — IBA (Ion Beam Applications SA) has received U.S. Food and Drug Administration (FDA) clearance for its new super conducting accelerator, which is the latest development, essential to the ProteusONE compact Intensity Modulated Proton Therapy (IMPT) solution, that required approval from regulatory authorities.
The FDA clearance makes ProteusONE, the only compact proton therapy solution in the industry, that is certified to treat patients in the United States with optimized pencil beam scanning (PBS) and image guidance.
IBA has already signed 13 contracts to provide its compact proton therapy solution worldwide. The FDA clearance is an important milestone that will ensure on-time delivery of all of IBA’s current and future contracts.
“FDA clearance of our ProteusONE's new super conducting accelerator will be a significant market accelerator for proton therapy in the United States, because ProteusONE provides the most advanced treatment mode within the most compact and affordable system,” said Beth Klein, president of IBA Proton Therapy North America. “Any hospital considering proton therapy can now afford it through the ProteusONE’s lower capital and operating costs without making any clinical compromise. Ultimately, this will make proton therapy more accessible to the radiation therapy patients in the United States who can benefit from it.”
The ProteusONE is the world’s only FDA cleared and CE marked compact solution that uses image guided IMPT. With IBA’s industrialized production together with full CE approval and FDA clearance, IBA is now able to install complete proton therapy system in less than 12 months.
The ProteusONE is equipped with pencil beam scanning, a technology that offers high sub-millimeter precision treatment, which allows delivery of very high levels of conformity and dose uniformity, even in complex-shaped tumors, whilst at the same time sparing the surrounding healthy tissue.
For more information: www.iba-worldwide.com


 February 04, 2026
February 04, 2026 









